From the very beginning of my research into virology’s claims, my priority has been to examine the foundational evidence for the existence of “pathogenic viruses.” Rather than relying on external critiques, I chose to analyze virology’s own literature, exposing its pseudoscientific methods using the field’s own work. My approach has been to highlight the internal flaws and logical inconsistencies in virological research, demonstrating that its conclusions fail to meet essential scientific standards. The experiments had already been conducted, and the supposed “evidence” was already documented—I simply needed to expose how it failed to support virology’s claims. As Kary Mullis, the inventor of PCR, emphasized, challenging a scientific claim is ultimately about logic.
One does not need to be a virologist, work in a lab, or conduct experiments to critically evaluate the evidence behind a hypothesis. The key question is whether the foundational research adheres to the scientific method and provides logically sound evidence. If it does not, the hypothesis is invalid. The burden of proof lies entirely with those making the claim, meaning that anyone asserting virology’s conclusions as scientific fact must either produce valid evidence or acknowledge its absence.
This approach has been highly effective for myself and others working to expose the flaws of virology. Every aspect of this pseudoscientific field—from failed contagion studies to flawed cell culture experiments—has been systematically refuted using virology’s own sources. Through simple logic, we have demonstrated that the scientific evidence virology claims to have does not actually exist within its own literature and that the “viral” hypothesis has been repeatedly falsified. Their burden of proof remains unmet.
Yet some, while superficially acknowledging that virology lacks the necessary scientific evidence, take an unusual stance: they argue that the burden does not fall on virologists to prove their methods meet scientific standards. Instead, they insist that identifying the logical and methodological flaws in virology’s literature is not enough—that those challenging virology’s claims must also conduct laboratory experiments to disprove them. In other words, they believe we must perform our own cell culture and electron microscopy studies to expose the methodological fraud in virology’s conclusions.
This is a subtle yet clear example of a burden of proof reversal fallacy—demanding that we experimentally prove a negative (i.e., that “viruses” do not exist) while using the very methodologies that presuppose their existence. While additional evidence can always strengthen a case, it is unnecessary when nearly 150 years of virology studies contain sufficient contradictions and methodological flaws to refute the “viral” hypothesis.
For example, the cell culture experiments used to “prove” the existence of “viruses” have already been dismantled through logical analysis and a critical review of virology’s own literature. No hands-on laboratory work was required to expose these flaws—just a clear understanding of the scientific method. Since these experiments do not originate from a naturally observed phenomenon leading to a testable, falsifiable hypothesis, do not begin with an independent variable that is isolated and manipulated, and rely on an artificial lab-created effect with multiple known “non-viral” causes, they fail to meet the standards of genuine scientific inquiry. Therefore, they have already been demonstrated to be pseudoscientific without requiring anyone to step into a lab.
Could we commission labs to replicate these experiments and reaffirm what virology’s own literature has demonstrated since John Franklin Enders introduced the cell culture method in 1954? Certainly. But is it necessary? Not at all. Virologists themselves have already revealed the pseudoscientific flaws in their own work. This is precisely why, during the height of the AIDS epidemic in the 1980s, HIV/AIDS dissidents like the Perth Group did not need to conduct their own lab work to expose the logical and scientific flaws in the claim that a novel “retrovirus” caused a new syndrome. They had an arsenal of flawed evidence from peer-reviewed journals at their disposal, along with a firm grasp of logic and the scientific method to dismantle the prevailing narrative. Their rigorous analysis of virology’s own literature laid the foundation for challenging the HIV/AIDS paradigm, providing a strong basis for future scrutiny of virological claims.
However, since some may continue to argue that engaging with virology’s flawed methodologies is necessary to expose its errors, I want to provide a detailed example of why this is ultimately unnecessary. Since the flaws of cell culture experiments have already been thoroughly exposed, I will turn to electron microscopy (EM) images of “virus-like” particles as another key example. Although the validity of these images has already been questioned—due to flaws in EM preparation methods and the misidentification of normal cellular structures as “viruses”—I will take an even deeper look at the foundational evidence to further reveal the fraud. I will begin with a brief history of the first use of EM to identify particles assumed to be "viruses," then examine key studies from the 1930s to the 1980s. I will demonstrate that these “virus-like” particles have never been scientifically validated as “pathogenic viruses” and that identical structures have been routinely observed in tissue and cell cultures labeled as “virus-free.”
This analysis will demonstrate that exposing the fraudulent nature of these images does not require becoming an electron microscopist or hiring one. Having a clear understanding of logic and the scientific method to critically examine the existing evidence is more than enough.
Before the invention of the electron microscope by German engineer Ernst Ruska in 1931, “viruses” were purely theoretical entities that were inferred rather than observed. Their existence was assumed based on passing materials from diseased hosts through filters designed to retain bacteria and being able to cause experimental disease in animals. Since “viruses” were too small to be seen with light microscopes, their nature remained an elusive mystery. As virologist Karen Scholthof noted in a personal communication with historian Tor Van Helvoort, for decades, “viruses” remained “outside the bounds of understanding. We couldn’t see them.”
In 1932, just a year after the electron microscope was invented, virologist Thomas Rivers characterized these invisible agents in negative terms through three defining characteristics: they could not be seen under a regular microscope, they passed through filters that trapped bacteria, and they could not reproduce without living cells. At the time, scientists debated what a “virus” actually was—some thought it was a toxin, an enzyme, a ferment, a soluable substance, a high-molecular-weight protein, or an exceptionally small microorganism. The definition remained fluid for much of the early 20th century, and the term “virus” was broadly used to refer to any “infectious agent” until the mid-20th century, when advances in electron microscopy and molecular biology manufactured the consensus that “viruses” were distinct particulate entities.
With the advent of the electron microscope in 1931, virologists could finally “see” the presumed entities that had remained invisible under light microscopes, which were limited to magnifications of around 1,000 times. Now, with magnifications ranging from 10,000 to 50,000 times, they could visualize individual particles that had previously existed only as theoretical constructs. As one observer put it, the electron microscope granted scientists “the power to actually see as individual objects things that had been only mental concepts.”
This technological breakthrough marked a paradigm shift. Until then, “viruses” had been identified primarily by their ability to cause disease in hosts. Now, they would be classified based on the morphology of the particles observed. Historian Tor Van Helvoort emphasized the significance of this shift, stating that the electron microscope was “first among the equals” of all the technologies that transformed the concept of the “virus.” He noted that it was “no coincidence that virology made its break from bacteriology and acquired the status of an independent discipline only after this instrument was invented and the first viruses were made visible.”
But did the ability to visualize once invisible particles with electron microscopy truly confirm their identity as pathogenic “viruses?” To answer that, we must examine the earliest depictions of these structures and evaluate whether the assumptions hold true.
Rockefeller man Frank MacFarlane Burnet, known for his work on polio and “antibodies,” is credited with being the first to use the term “virus-like particles” in his 1933 paper on the neutralization of bacteriophages by antiphage serum.
Specific Agglutination of Bacteriophage Particles
The photographs in themselves do not convey a great deal of information, and it is conceivable that under certain conditions specific precipitates from soluble reagents might produce aggregates of similar microscopic appearance. But on the other hand, if virus-like particles of the size found for phage C16 by filtration methods 50-75 mu were aggregated into masses by specific serum agglutination, then one would expect that the image obtainable with the available technique would be exactly similar to what is in fact found. It is not possible to give any accurate estimate of the diameter of the phage particles from these photographs, but they indicate that it is probably not much more than 50 mu.”
https://pmc.ncbi.nlm.nih.gov/articles/PMC2048370/
The image presented in the paper was not derived from electron microscopy but from ultraviolet microphotographs. Rather than depicting individual “virus-like” particles, the images showed amorphous or aggregated material. No scientific evidence was presented to confirm that the structures identified in these microphotographs were actual pockets of “viral” entities.
Despite this uncertainty, Burnet not only classified the invisible bacteriophages as “viruses” of bacteria—an assertion still heavily contested at the time—but also took the additional step of declaring that “viruses” were particulate entities. While virologists like Thomas Rivers had also referred to “viruses” as particles, this remained a significant assumption, as there was still no consensus on whether a “virus” was a discrete particle at all or merely a poisonous substance of some kind. In fact, researchers Vinson and Petre in 1931 described the tobacco mosaic “virus” behaving like a chemical, stating, “…it is probable that the virus which we have investigated reacted as a chemical substance.” Their conclusion was reinforced in 1933 by Barton-Wright and McBain, who, after replicating their work, affirmed, “We have repeated this work in detail and confirmed it in every particular, and we are also of the opinion that the virus in this case behaves as a chemical compound and not as a living organism.”
Regardless, with the emergence of technology capable of “seeing” nanoparticles, the search for the elusive “virus” particle began. Early electron microscopy studies examined cellular debris—which many argue is all that “viral” particles truly are—as well as material from chicken egg cultures, which had began being used in the 1930s to claim that these supposed “viral” entities had been cultivated and grown. Interestingly, it was not Ernst Ruska but his brother Helmut who first utilized the electron microscope to identify “virus-like” particles. His 1938 paper Bakterien und Virus in Übermikroskopischer Aufnahme presented electron microscope images of particles he attributed to the “poxvirus” (vaccinia), mouse ectromelia “virus,” and rabbit myxoma “virus.”
The first image from his paper depicted spherical particles claimed to be the mouse ectromelia “virus.” The “infectious” material was sourced from the lymph of a diseased mouse paw and magnified 20,000 times. However, there was no explanation as to how these specific particles were identified as the “virus” in question, let alone as a “pathogen,” rather than normal cellular constituents or debris.
“Fig. 1. Supermicroscopic image of the white mouse ectromelia virus. Infectious material from the lymph of a diseased paw. Electron-optical magnification: 20,000.”
“The supermicroscope can be used to make visible pathogens that were previously invisible in the light microscope due to their small size without the use of staining methods. Fig. I shows the mouse ectromelia virus in a supermicroscopic image. It can already be predicted with certainty that even those pathogens that have so far evaded morphological detection will be imageable.”
Helmut mentioned that the electron microscope could now visualize “pathogens” too small for light microscopy, which he believed included bacteriophages and “viruses.” However, his presumption about the size of the “virus” led to a confirmation bias, as he focused on particles in a specific size range, already assuming they were “viral.” While he claimed to have imaged “viruses” like smallpox, ectromelia, and myxoma, Helmut acknowledged a critical issue: “The objection could be made that our newly discovered structures are artificial products, created by the vacuum or electron beams.”
Helmut's study was driven by the assumption that “elementary bodies of viral diseases” could be prepared and imaged as easily as bacterial structures. This assumption-based approach, rather than a purely empirical one, led to the use of biological materials from the State Vaccination Institute, which were then transferred through rabbits and chicken egg allantois. The use of living tissues in sample preparation introduced confounding variables, such as cellular debris and other biological structures, which could then be misidentified as “viral” particles. Helmut noted that the electron microscope images deviated from prior visual representations (likely artistic interpretations or light microscopy), showing a clear discrepancy between earlier depictions and actual observations.
Furthermore, the use of glycerine in the preparation process resulted in blurred, washed-out images, which introduced additional artifacts and further complicated the interpretation of the data.
“After the discovery of the smallest accompanying bodies of bacteria, it was expected that elementary bodies of viral diseases could also be prepared without difficulty. Fig. 21 shows Paschen's bodies, the elementary bodies of the vaccine. The starting material was kindly provided to us by the State Vaccination Institute and was then transferred to rabbits and the allantois of the incubated chicken. We were unable to find the forms drawn by KRAUSE from the electron optical image. The image shown is blurred and washed out due to the preparation of the virus in glycerine.”
Helmut acknowledged that the preparation of the material with saline led to the observation of “small square structures of various sizes,” which were attributed to salt crystallization, rather than “viral” particles themselves. Only after removing the salt were the “elementary bodies” observed in their “true size and shape.” This suggests that what was being visualized may not have been distinct biological entities but artifacts from the sample preparation. While Helmut claimed that the preparations were “proven infectious in animal experiments,” this does not confirm that the imaged structures were “viruses”—it only meant that the material caused symptoms in animals, not necessarily due to the particles observed under the microscope.
Helmut’s description of some “virus” particles as “egg-shaped” rather than spherical further underscores the subjective nature of the “virus” classification process. This variability raises concerns about the interpretation of the images and the potential influence of preparation artifacts.
“We therefore switched to transferring the virus to saline solution when depicting ectromelia. The success of this procedure can be seen in Fig. 22. The preparations obtained showed small square structures of various sizes. Round structures, such as we had expected to be elementary bodies, were not visible, although the preparations proved to be infectious in animal experiments. The phenomenon can be interpreted as the salt around the elementary bodies crystallizing out as a crystallization center when the preparation dries. The square structures are cubes of table salt, each of which encloses an elementary body. Only when the table salt is removed can the elementary bodies be seen in their true size and shape in an uncolored state, as shown in Fig. 23. Here too, individual elementary bodies are covered by a salt crust, which makes them stand out from others in the image due to their stronger blackening. The preparations are checked by staining according to HERZBERG and by mouse experiments. The last picture (Fig. 24) shows elementary bodies from rabbit myxoma disease. The concentration of the virus in the preparation was still so low that it was not possible to get several elementary bodies into one field of view at the same time. In the picture you can see how clearly the virus stands out from the remaining amounts of protein that adhere to the preparation and you can see that it has an egg-shaped shape. Such deviations from the spherical shape offer the prospect of a differential diagnostic distinction between different elementary bodies.”
From this initial paper, it is clear that early electron microscopy studies of presumed “viruses” were hampered by subjective interpretation, preparation artifacts, contamination, and reliance on indirect evidence such as “infectivity” in animals. Identifying “viral” particles was not straightforward, and the results were heavily influenced by experimental conditions, casting doubt on whether the observed structures were truly distinct “viral” entities.
No attempt was made to purify and isolate the assumed “virus” from either the host or the cultures to demonstrate pathogenicity using the scientific method. Instead, Helmut Ruska relied on morphology and size to identify presumed “viral” particles—an approach that set a precedent in virology that has been followed for nearly a century. This practice established a subjective framework in which cellular debris, artifacts, and other structures could be interpreted as “viruses” based on assumed characteristics rather than direct evidence of causation.
The uncertainty surrounding electron micrographs of presumed “viral” particles was summarized by Van Helvoort, who noted that there were “doubts aplenty” about what these images actually showed or meant. Upon seeing the first EM images, eminent Belgian microbiologist and immunologist Jules Bordet lamented that it was “troublesome enough to interpret the images” from regular light microscopes. Biophysicist and virologist Robley Williams acknowledged that “the world is embarrassingly full of tiny objects that look alike” and admitted that “any photograph contains objects that one might have anticipated,” underscoring the preconceived subjectivity involved.
Interpreting electron micrographs was further complicated by artifacts introduced during the various preparatory stages of specimen processing. Since “viruses” were an unknown structure, distinguishing genuine “viral” particles from artifacts became a critical yet highly uncertain task. Van Helvoort noted the high likelihood of mistaking artifacts for “viruses” and observed that some artifacts even took on a life of their own, temporarily becoming accepted as scientific objects in their own right—an argument that could still be made today.
Without clear indices for comparison, particles presumed to be “viruses” were “easily confused with other material that may exist in their environment.” Even Helmut Ruska himself struggled to differentiate between bacteriophages and components of bacterial cells.
How Seeing Became Knowing: The Role of the Electron Microscope in Shaping the Modern Definition of Viruses
“Even after the initial success in obtaining images, there were doubts aplenty about what they actually showed or meant. ‘‘Oh no, no! Let us not have an electron microscope – it’s troublesome enough to interpret the images we get by the light microscope,’’ the illustrious Belgian microbiologist and immunologist Jules Bordet is quoted as having said at a colloquium where the first biological electron micrographs were presented (Marton, 1976, p. 282). ‘‘Practically any photograph contains objects which look like something which one might have anticipated!,’’ grumbled Robley Williams, referring to the difficulties in understanding images, especially in the early years, observing as well that ‘‘The world is embarrassingly full of tiny objects which look alike, and which carry no printed labels!’’ (1947, p. 213). The tiny viruses posed special problems of this sort in the early years of electron microscopy. There being no indices for comparison, they were ‘‘easily confused with other material that may exist in their environment’’ (Hillier, 1950, p. 3). When, for instance, Helmut Ruska first observed several ‘‘sperm-shaped’’ particles in the electron micrographs of bacteriophage preparations, he was uncertain as to whether these particles were bacteriophages or constituents of the bacterial cell (1941).”
“As if the identification was not difficult enough, scientists became aware of even more serious problems in interpreting electron micrographs due to artifacts that were introduced in the images during various preparatory stages of the specimen (Marton, 1976, p. 293). It was only in the study of known elements that artifacts did not play an important role, ‘‘because of the rigidity and stability of most of the structures in question’’ (Hillier, 1950, p. 3). In the case of viruses during these early years, there was a high chance of mistaking artifacts and viruses for each other. Indeed, some artifacts even acquired a life of their own, becoming for a time scientific objects in their own right; see, for example, Rasmussen’s story of the so-called bacterial mesosome (1993).”
https://pubmed.ncbi.nlm.nih.gov/29926225/
With the invention of the electron microscope and Helmut Ruska’s early images—asserted to depict “pathogenic viruses” without scientific evidence demonstrating as much—the stage was set for the “virus” hunters to spend the following decades searching for similar particles to blame as disease-causing agents, even as these same particles were found in places where they shouldn't be while causing no harm.
In 1943, Helmut Ruska proposed that all “viruses”—regardless of whether they were associated with vertebrates, bacteria, or plants—should be classified based on their morphology. This marked a major departure from previous classification methods, which were based on the ability of these invisible entities to cause disease. As historian Van Helvoort noted in his 2018 paper How Seeing Became Knowing: The Role of the Electron Microscope in Shaping the Modern Definition of Viruses, the introduction of the electron microscope meant that “viruses” were no longer just hypothetical, invisible disease agents. Instead, researchers could now detect particles assumed to be “unknown viruses” in various samples, even in cases where there were no signs of disease.
In his 1943 paper Versuch zu einer Ordnung der Virusarten, Ruska argued that “viruses” should be classified primarily by their structure as observed in electron microscopy (EM) images. While he acknowledged that many fundamental questions about “viruses” remained unanswered, he stated that researchers could “deliberately dispense with clues provided by the reaction of the diseased organism” when classifying them. This shift is significant because it meant that “viruses” were being categorized based on their appearance alone, rather than through experimental proof of their ability to cause disease. Instead of fulfilling Koch’s Postulates and following the scientific method to establish causation, researchers assumed that certain particles were “viruses” based solely on their structure.
“However, even if these basic questions have not been resolved, an attempt can be made to classify them by establishing related groups. In doing so, we deliberately dispense with clues that are provided by the reaction of the diseased organism and only subject the pathogenic agents to a comparative examination.
Ruska emphasized that morphology is a key factor in biological classification and argued that electron microscopy allowed for more precise measurements of “virus” particles compared to light microscopy. However, he also assumed that morphology was directly linked to biological function and “pathogenicity”—despite recognizing methodological limitations. This is an example of affirming the consequent, a logical fallacy in which a shared characteristic is taken as proof of identical function. In other words, researchers assumed that because certain particles had a specific shape, they must be the same type of entity with the same role in disease.
In biology, we primarily use morphological characteristics as a basis for systematic ordering. They will also allow us to find groups that belong together in virus research, since the size and shape of the contagions can be measured much better than before using light microscopy. The internal structure and the surface properties will have to be assessed using other methods based on the characteristics that have been shown by sub-light microscopic morphology and structural research. In addition to morphology, the biological structure and physiological behavior, especially the mode of reproduction, are important. In the case of structures without a nucleus or subcellular structures, it is closely linked to the character of the substances that are decisive for the constant biological properties. According to our current knowledge, these are particularly the nucleoproteins. For each virus, the morphological characteristics include the size, shape, structure and surface properties, and the physiological characteristics include the type of metabolism, growth and reproduction, as well as the occurrence of nucleoproteins. Even if there are still narrow methodological limits to the implementation of these requirements, a rational classification of virus types will still result on the basis of these characteristics. Their choice does not mean a change in the aspects that are valid today in every systematic order, but rather their consistent continuation.
Importantly, Ruska admitted that many classification criteria for “viruses” were either unknown or simply estimated rather than directly observed. This means particles were being labeled as “viruses” even in cases where essential biological properties had not been “confirmed.” Some classifications were made based on what could not be demonstrated, rather than what could be conclusively proven—a serious flaw in scientific reasoning.
Furthermore, Ruska grouped bacteria and protozoa within the same classification framework as “viruses,” drawing comparisons between them despite lacking independent evidence that “viruses” could replicate, metabolize, or cause disease on their own. Rather than proving that these particles were biological entities, Ruska presupposed that they were. His approach ultimately led to the classification of “viruses” based on assumptions rather than direct experimental evidence.
If we classify the contagions for which we know or are able to estimate some of the criteria listed, we inevitably end up in groups that belong together, and every virus for which the necessary documents have been found can be described in a corresponding manner. Up to now, the information entered in the table can be given. It is partly formulated negatively, for example by stating that this or that morphological peculiarity or physiological ability could not be proven. For reasons of comparison, bacteria and protozoa are also listed among the contagions.”
Ruska noted that, at the time, most “viruses” were assumed to be spherical based on indirect measurements from light and ultraviolet microscopy. However, he pointed out that this assumption might be incorrect, stating that “elementary bodies that always show a spherical shape have not yet been imaged.” This underscored the reliance on assumptions and indirect methods rather than direct observation in defining the morphology of presumed “viruses.” Virologists had been working with hypothetical models rather than actual observed structures. When direct imaging finally became possible, it contradicted previous assumptions, revealing that the field had been operating on presumed “virus” shapes rather than “confirmed” ones. If “viruses” were real, distinct entities, why did their basic morphology have to be inferred rather than directly observed?
“So far, the shape of most viruses is considered to be round (“strongyloplasms”, Lipschiitz). The first finding to deviate from this was the observation of the flow double refraction of solutions of the tobacco mosaic virus protein, from which it was concluded that the virus particles are rod-shaped.
The image by means of supermicroscopy confirmed this assumption and further showed that the idea of the round shape of many viruses obtained for other elementary units from light and ultraviolet microscopy may be incorrect. Elementary bodies that always show a spherical shape have not yet been imaged electron-optically.”
Ruska’s own words reveal that “viruses” were being classified as “pathogenic agents” based purely on morphology, without scientifically establishing their causal role in disease. His work highlights how virology has been entrenched in circular reasoning and flawed assumptions from its inception. Rather than relying on rigorous scientific proof, virology has historically depended on inference and assumption to assert that the particles observed in EM images are “pathogenic viruses” rather than normal host components, cellular debris, or artifacts from sample preparation. By classifying particles as “viruses” based solely on their appearance, Ruska’s approach opened the floodgates for “virus-like” particles to be identified nearly everywhere—despite the absence of scientific evidence confirming their “pathogenicity.”
Many of the earliest reports of these “virus-like” particles outside of Ruska's came from studies of cancer cells or tumor-derived cell lines in the 1940s. Researchers claimed that the particles they observed resembled those seen in electron micrographs of supposed animal “viruses” as described by Ruska and others.
For example, in a 1947 paper by Claude et al., chicken tumor cells were examined using the newly emerging electron microscopy technology. They reported that filtration experiments had estimated their assumed invisible “agent” to be approximately 70 mu in diameter. In practical terms, this meant that they passed unpurified tumor fluids through progressively smaller filters while injecting animals with the filtrate. If the animals became ill, it was inferred that an “infectious agent” was present and small enough to pass through that particular filter.
Although the researchers claimed that their preparations were of “high potency”—purportedly concentrated and purified (i.e., free of contaminants)—they acknowledged that the samples were contaminated with a normal, submicroscopic cytoplasmic constituent of nearly the same size as the presumed tumor agent. Consequently, their “purified” fractions, when examined under dark-field illumination, showed large amounts of this normal contaminant, rendering their observations inconclusive.
Light microscopy had already failed to reveal the agent because it was too small to be resolved, and initial investigations did not show any “inclusion bodies” (the nonspecific, indirect markers associated with “viral infections”) or other abnormalities in the tumor cells. With these methods proving inadequate, the researchers turned to electron microscopy. They then observed small bodies—of the size predicted from centrifugation and filtration tests—that were present in tumor cells but absent in normal cells obtained from adult fowls, chick embryos, or the buffy coat of chicken blood. Based on this selective presence and the matching size, Claude et al. considered the evidence “strong” that these small bodies were indeed their invisible tumor agent.
Electron Microscope Study of Chicken Tumor Cells
“Chicken tumors of spontaneous origin are characterized by the fact that most of them can be transmitted to new hosts in the absence of cells (11). From the beginning it was apparent that the agents transmitting the tumors were of relatively small size since they were not retained by Berkefeld filters of medium porosity (30, 31, 32, 33). On the basis of filtration experiments using collodion membranes of graded pore sizes it was estimated that the probable diameter of the agent of chicken tumor I was approximately 70 mu (15). A close agreement with this value was obtained from determination of the speed of sedimentation of the tumor-producing factor in high centrifugal fields (6, 16, 20). Concentration and purification of the agent by means of differential centrifugation gave preparations of high potency (6, 7). Subsequently, it was found that these active fractions were contaminated with a normal, submicroscopic, constituent of cytoplasm of nearly the same size as the tumor agent (8).
Because of their small size, the agents causing chicken tumors cannot be resolved by means of light microscopy; purified fractions containing them have appeared particulate under dark-field illumination (6) but the presence in large amounts of a normal contaminant made these observations inconclusive. The light microscope has also failed to disclose any inclusion bodies or other abnormalities in the tumor cells that might be considered as indicative of the presence of the filterable agent.
In the present investigation advantage has been taken of the fact, demonstrated in an earlier study (28), that cells of most types in tissue culture spread out to a thinness which makes electron microscopy possible. Chicken sarcoma cells are among the elements which behave in this way. A detailed study has now been made of those from two tumors, namely Chicken Tumor I and Chicken Tumor 10. The electron microscope has revealed in the cytoplasm of these cells, small bodies of a size predicted from centrifugation and filtration tests with the tumor agents. The disposition of these bodies within the cell appears to be distinctive for the kind of tumor examined. No similar structures have been found in an extensive study of homologous normal cells obtained from tissues of adult fowls or from chick embryos, or from the buffy coat of chicken blood. The evidence is strong that the small bodies in the neoplastic cells are the individual tumor-producing entities.”
“In addition to those constituents of normal cells illustrated in Fig. 1, chicken tumor cells have been found to contain in variable abundance small bodies, characteristic in size and appearance, which are considered to represent the causative agents of the disease. Such bodies have not been detected in normal chicken cells thus far examined by electron microscopy.”
“The micrograph shows the inclusions commonly found in normal cells, i.e., scattered Golgi or fat bodies, mitochondria, and constituents of the ground substance. In addition and of special interest is the presence of patches of greater density which vary in size and shape and which thus far have been detected only in cells of Chicken Tumor 10. It is readily seen in Fig. 2 that these patches result from the aggregation of small elements of uniform size and shape and which appear not unlike growing colonies of staphylococci. In the case of Chicken Tumor 10, it might be assumed that these formations have resulted from the growth and subdivision of their constituting elements. From measurements on enlarged micrographs the diameters of the individual bodies are found to vary from 60 to 70 m/s. The presence of small bodies of this size in tumor cells but not in normal cells, their arrangement in "colonies," a feature apparently typical for Chicken Tumor 10, support the conclusion that the small elements observed represent the causative agent of the tumor.”
“The outstanding feature of the micrograph illustrated in Fig. 3 is the presence of numerous and conspicuous small bodies disposed along the cell margin, which appear singly, frequently in pairs, or in small rows of 4 or 6. Considerations to be discussed later suggest that these small elements represent the causative agent of Chicken Tumor I.
Because the particles were found exclusively in tumor cells (and not in normal cells), matched the predetermined estimated size of a “virus,” and there was no evidence at the time of characteristics suggesting they were normal cytoplasmic constituents or products of abnormal cell activity, Claude et al. concluded that these were “extraneous entities” introduced from outside. They further noted that the particles occurred in pairs and exhibited variations in size, which led them to speculate that the particles “may be self-duplicating, possibly autonomous” and thus “possess properties commonly attributed to viruses.”
The disposition of the agent in pairs or in small loose groups seems typical for Chicken Tumor I. It is further illustrated in the next micrograph (Fig. 4). In this particular case, the body of the tumor cells in the tissue culture was removed, possibly during the preparation of the mount, leaving on the film adherent patches of the cell membrane together with series of bodies similar to those seen in the intact cell illustrated in Fig. 3. Measurements on enlarged micrographs indicate that the size of these bodies, in different preparations, ranges from 67 to 84 mu in diameter. Close examination of the preparation shown in Fig. 4 reveals that paired elements are numerous and probably more frequent than could be expected if the occurrence in pairs were determined by chance alone. In the present case it seems reasonable to assume that these bodies are capable of dividing and that their appearance in pairs and in short strings is the result of a single, or a few successive subdivisions. It is conceivable that the arrangement found in Chicken Tumor I cells would be achieved if the process of successive duplication of the chicken tumor agent were oriented in space according to one plane, whereas the disposition obtained in Chicken Tumor 10 could result from subdivision of the agent at random, or according to planes oriented, alternatively, at right angles to each other.”
Summary
1. The cells of Chicken Tumor I and Chicken Tumor 10 spread out so thin when grown in tissue culture that photographs can be taken with the electron microscope giving clear definition of the peripheral portions of the cytoplasm.
2. Small bodies, the size of that estimated for the transmitting tumor agents, are present in the chicken tumor cells but are not found in homologous normal cells.
3. The bodies observed in the cells of Chicken Tumor 10 lie side by side in patches often of considerable size, whereas the Chicken Tumor I agent is dispersed singly or else in pairs and only very occasionally in small groups.
4. No evidence has been found to support the view that the bodies are the homologues of normal cytoplasmic constituents or are the products of abnormal cell activities. They have all the appearance of extraneous entities. Their occurrence in pairs in the cells of Chicken Tumor I and their variations in sizes suggest that they may be self-duplicating, possibly autonomous. In all these particulars the chicken tumor agents appear to possess properties commonly attributed to viruses.
While Claude et al. produced images of small particles, they lacked scientific evidence—derived from the scientific method and satisfying Koch’s postulates—to prove that these particles were “pathogenic viruses” capable of inducing tumors. Their findings amounted to correlation, not scientific proof of causation. Like Ruska, they took unpurified materials from diseased tissues, assumed a “virus” was present, and then used EM to search for particles matching the predetermined morphological characteristics of what “viruses” were claimed to look like. Once they identified such particles, they simply declared them to be a “virus” responsible for causing tumors—without rigorous proof of “pathogenicity.”
A year later in 1948, Porter and Thompson used TEM to examine cells from mammary tumors of mice. They cited earlier work suggesting that these tumors were influenced by a “transmissible agent” with “many of the characteristics of a virus.” Since the agent was invisible, they noted that two previous attempts had been made to study it using electron microscopy to define its nature more precisely. In the first examination of a “high cancer strain,” a “heavy particle” was identified, which had “virus-like dimensions.” The second examination found a particulate component, about 200 Å in diameter, which was absent in extracts from “low-cancer strains.” However, Porter and Thompson cautioned that both studies had been criticized for the possibility that the preparation methods had significantly altered the “agent” and that the particles might have been confused with normal cytoplasmic elements.
A particulate body associated with epithelial cells cultured from mammary carcinomas of mice of a milkfactor strain
“The occurrence of mammary tumors in mice has been shown to be influenced by a transmissible agent, by an inherited tendency to develop breast cancer, and by hormonal stimulation of the gland tissue. Since the initial report of the existence of an extrachromosomal factor (1) and the demonstration by Bittner of its presence in the milk (2), many investigators have sought a definition of its mode of action, its transmission, and its character. Their results have shown the agent to have many of the characteristics of a virus (3-5). In order to define the nature of the agent more precisely, attempts have been made to study it with the electron microscope. At the time of this writing, we are aware of only two previous accounts of such efforts. Graft et al. (6) have examined the ultracentrifugate of milk of high-cancer and low-cancer strains of mice. In a brief note they report that the high cancer strain milk contains a "heavy particle" which "has virus-like dimensions." Passey et al. (7) made water extracts of desiccated normal and malignant breast tissue from mice of high- and low-cancer strains. In micrographs of material from high-cancer strains they found a particulate component about 200 A in diameter which, they report, was not present in extracts of tissue from low-cancer strains. Both of these observations, the latter more than the former, are subject to the criticisms that the agent may be greatly altered by the preparation procedures and may be easily confused in microscopy with particulate elements present in the cytoplasm of all cells. A study of the cells themselves would be less subject to these criticisms and might, moreover, give some information on the mode of reproduction of the agent and its relation to the tissue cells.
Porter and Thompson noted that their own research had demonstrated the suitability of cultured cells for electron microscopy, making it a viable method for identifying “viruses” in or on cells. However, it should be noted that they had just mentioned that earlier TEM results might have been skewed by the preparation procedures alone. Their method involved using cells grown from explants of both spontaneous and transplanted tumors. The cells were cultured in a medium consisting of equal parts nutrient and chick plasma, diluted 1:4 with Tyrode's solution, and other components like human cord serum and chick embryo extract. Cultures were made from tumor tissue of a single animal, with variations in the serum/extract used. After a few days of culture, explants were removed, washed, and fixed in osmium tetroxide vapor. How they ensured that their method did not alter the presumed “agent” remained unaddressed.
Earlier reports from this laboratory have disclosed the suitability of cultured cells for electron microscopy (8) and have demonstrated that the method can be used not only for the study of new cytological detail (9) but can be applied as well to the identification of viruses in or on cells (10) and to the study of the special cytological features of malignant cells (11). It seemed worth while to use similar techniques in an investigation of the cells from mammary tumors of the mouse. The following is a report of the initial efforts.”
“The cells for study were grown from explants of both spontaneous and transplanted tumors. In all, four spontaneous and two transplanted tumors have been used. The former originated in female mice of the high tumor strain Call, a strain in which the milk factor is known to be operative; the latter were fifth generation transplants of a spontaneous tumor 1 that also; arose in a C3H mouse. All tumors were typical adenocarcinomas of the mammary gland that is to say they had the character of the growths known to be determined by the milk factor.
Cultures were prepared on formvar-coated slide inserts in roller flasks by methods described already (8). The explants were placed in shallow clots made up of equal parts of nutrient and chick plasma diluted 1:4 with Tyrode's solution. The nutrient was composed of 5 parts Tyrode's, 3 parts human cord serum, and 2 parts chick embryo extract. Each set of cultures was made with tumor tissue from a single animal, and successive sets were separated by intervals of 2 weeks or more. Different lots of cord serum, plasma, and embryo extract were used on each set. When, after a few days of culturing, small sheets of epithelial cells were obtained, the explants were removed, the remaining cells were washed briefly in a slow stream of Tyrode's (pH 7.4) and then placed in the vapor of osmium tetroxide for fixation. After periods over OsO4, varying from 2 to 24 hours, the cells were mounted on screens and dried for electron microscope examination. All the micrographs were taken with an RCA (type E.M.U.) instrument.”
Upon examination, Porter and Thompson observed a number of small “apparently spherical” particles associated with some cells. Yet, in three out of their six experiments, the particle described was not found. Porter and Thompson argued that this did not prove the particles were absent, just that they were not found through their examination. The particles appeared similar in size, and possibly in other aspects, to normal cytoplasmic granules. Nevertheless, they suggested that the particles’ appearance in clumps and their irregular occurrence in tumor cells made it likely they were of “extraneous origin” and potentially “viral.”
“The electron microscope examination of preparations of the epithelial sheets had not gone far before it was observed that numbers of a small, apparently spherical particle were associated with some of the cells (Fig. 4). The characteristic density and morphology of these particles set them off from the normal cytoplasmic components (Figs. 5 and 7). In some cells they lay scattered in small numbers (Figs. 4 and 5) while in others they literally packed the cell (Fig. 3).
“Table I lists the origins of the material examined and shows the observed occurrence of the particles. It will be noted that in the preparations obtained from three out of six experiments none of the special sort described has been found. This does not prove that none was there, it means only that none was encountered during a moderately extensive examination of the screens. In the single preparation of transplanted tumor cells in which the particles were noted they were found only over a small area of the screen, in connection with a few cells.”
“These studies have disclosed the frequent presence in mouse mammary tumor cells of a particulate body, having a fairly uniform size, density, and morphology. Though similar in size, and possibly in other respects, to some normally occurring cytoplasmic granules (11), these particles are sufficiently different from the latter to give the impression of being special entities. Their uniform morphology, their association in closely packed clumps, as well as their irregular occurrence in tumor cells are especially significant as features distinguishing them from normal components of the cell. These same features make it seem probable that they are of extraneous origin and that they may be a virus.”
In a moment of honesty, the researchers admitted that they could not definitively determine if the particles represented the “milk agent” of mammary tumors. They concluded that there appears to be no simple and direct method for verification. Despite this, Porter and Thompson reported that they had developed the ability to discern abnormal particles from normal ones, granting them the ability to identify “virus-like” bodies in tissue cells.
“The most obvious, and for the moment, important question arising from these observations is whether or not the particles represent the milk agent of the mammary tumors. At present, there appears to be no simple and direct way of determining this; it will be necessary, instead, to gather evidence tediously through a comparison of tumor cells obtained from growths presumably carrying the milk agent with cells from the mammary tumors of agent-free animals.”
“Finally, it should be mentioned that a significant gain from these studies and our examination of normal cells, has been the experience needed for the recognition of virus-like bodies associated with tissue cells. As one acquires the ability to discriminate the unusual or abnormal, the prospect develops that yet more pathological material can be examined with profit.”
https://pmc.ncbi.nlm.nih.gov/articles/PMC2135805/
These examples from the late 1940s illustrate how researchers used electron microscopy to search for particles that fit their preconceived idea of what a “virus” should look and act like in diseased tissues. The practice of classifying such particles as “viral” based solely on morphology traces back to Helmut Ruska’s earliest EM images—despite the lack of scientific evidence proving these particles were pathogenic “viruses” to begin with. This marked the beginning of a circular process in which electron microscopy became a tool of self-reinforcing deception that carried on into the proceeding decades.
In 1950, a committee of virologists convened at the Fifth International Congress for Microbiology in Rio de Janeiro to establish a classification system for “viruses.” According to virologist Christopher Andrewes’ (of the Common Cold Unit notoriety) 1951 recollection, the committee unanimously decided to place morphology—rather than symptoms—at the forefront of classification. Previously, symptoms had been the defining characteristic of a “virus,” but they were now relegated to the bottom of the list.
First we considered what were the criteria to be applied in classifying viruses, and we were unanimous that these should in the first place be the properties of the virus itself, and that symptoms produced in the host should, with emphasis, be placed at the bottom of our list. The 8 criteria thought to be important were as follows: -
Morphology and methods of reproduction.
Chemical composition and physical properties.
Inimunological properties.
Susceptibility to physical and chemical agents.
Natural methods of transmission.
Host, tissue and cell tropisms.
Pathology, including inclusion-body formation.
Symptomatology.
As Van Helvoort noted, this was a striking reversal. Less than a dozen years earlier—before the electron microscopy boom—the American Phytopathological Society had listed symptoms as the primary criterion for classifying “viruses,” with physicochemical properties ranked last. Andrewes and his colleagues’ decision demonstrated the profound impact of electron microscopy, which not only influenced detection and identification but also fundamentally reshaped classification of the assumed “virus” particles.
This decision – reached, as it happened, by the ‘‘uninterested’’ animal virologists – was quite the volte-face, for less than a dozen years earlier a committee of the American Phytopathological Society had listed symptoms as the first criterion for classifying the viruses while physicochemical properties were last (Bennett, 1939, as cited in Lwoff and Tournier, 1966, pp. 48–49). This drastic turnabout in the hierarchy of criteria for virus classification demonstrates the central role that the electron microscope played in the paradigm shift to the modern definition of viruses. A perusal of viral classifications devised after the 1950 guidelines were set by Andrewes and others does, in fact, show an increasing reliance on the new looking glass, not only for the purposes of detection and identification, but also specifically for classification (see, for example, Wildy, 1962; Khanna and Lund, 1967). The two purposes even melded together on occasion; an electron microscopic investigation into the rabbit fibroma virus for instance, showed its morphology to be identical of two other groups of viruses – the rabbit myxoma virus and various pox viruses (Lloyd and Kahler, 1955). Thus morphology, specifically what the electron microscope revealed about the size, shape, and symmetry of the protein subunits of viruses, became one of the major characteristics by which viruses were grouped (Horne, 1964)."
During the 1950s, as tissue and cell cultures became more common practice, electron microscopy images of particles from these cultures began to increase rapidly, with researchers observing “virus-like” particles seemingly wherever they looked. One notable example occurred in 1956, when Fawcett used electron microscopy to examine materials from spontaneous tumors in four Northern leopard frogs. This work was built on earlier findings by Lucke, who had suggested the presence of an invisible “agent” linked to these tumors. Lucke made this conclusion based on the discovery of nonspecific inclusion bodies in the tissues, which were attributed to “viruses,” as well as his ability to induce tumors in the frogs by injecting desiccated or glycerinated tumor preparations. This led Lucke to hypothesize that the tumors were probably caused by an invisible species-specific, organ-specific “agent” having the characteristics of a “virus.”
Electron microscope observations on intracellular virus-like particles associated with the cells of the Lucké renal adenocarcinoma
“Relatively few malignant neoplasms have been described in amphibia. The one that has been studied most extensively is the Lucke renal adenocarcinoma of the frog, Rana pipiens. This malignant tumor occurs in from two to four percent of frogs of this species, collected in the Lake Champlain region of northern Vermont and adjoining areas of Quebec. In a series of papers Lucke described its histopathology (17), reported the occurrence of distant metastases (18) and demonstrated the presence in some of the tumors of intranuclear inclusion bodies of a kind commonly seen in virus diseases (17, 22). It was subsequently shown by him that tumors could be induced in frogs of the susceptible race by injection of desiccated or glycerinated preparations of tumor. Similar inoculation of other races or species was without effect. These observations led Lucke to conclude that the tumors were probably caused by a species-specific, organ-specific agent having the characteristics of a virus (19, 20). Additional supporting evidence for this conclusion was provided in later work of Schlulnberger and Lucke (31) in which the tumor was carried for many generations by serial intraocular transplantation. In the course of these experiments it was noted that frogs bearing intraocular tumors very often developed kidney tumors. Similar results have been reported by Rose and Rose (30) in anterior chamber transplants in adult frogs, and by Briggs (4) in tadpoles with tumor implants in the tail fin. In both instances persistently growing adenocarcinomas frequently developed in the kidneys even though the implanted tumor tissue itself regressed. The most reasonable interpretation of these findings seems to be that a tumor agent passed from the implanted tissue to the kidney and there induced a tumor of the same kind. Further evidence of the viral nature of the tumor-inducing material has been provided by the work of Duryee and Doherty (8, 9) who have recently established its filtrability.
In the present investigation, thin sections of frog renal tumors have been examined with the electron microscope. In some specimens the cells were found to contain virus-like particles of uniform size and distinctive form, which may be the postulated tumor agent. Sundry observations have been made on the distribution of these particles within the cells, on their internal structure and their mode of development.”
Fawcett, in his 1956 study, acknowledged that the particles he observed had a “striking resemblance” to those found by Morgan in 1954 in herpes simplex “virus-infected” cells. However, he was careful to note that the resemblance alone did not prove they were “viral.”
“Because of the striking resemblance of the particles described here to particles found by Morgan and his collaborators in cells known to contain virus of herpes simplex (25) we take the liberty of referring hereafter to these encapsulated spherical bodies as "virus particles" realizing that their viral nature is not established by such morphological resemblances alone. 2”
In reference number 2, Fawcett further acknowledged the lack of proof that the particles observed by electron microscopy were definitively “viruses.” He explained that, despite this uncertainty, the term “virus-like” particles was adopted, yet he believed little was gained by describing them as “like” other particles found in “virus-infected” cells when the “viral” nature of those particles had not been definitively shown. As a result, he chose to simply refer to the observed particles as “virus particles” for simplicity and readability, hoping that readers would understand his reservations.
Proof that the various particles that have been described in electron micrographs of virus infected cells are actually the virus is still lacking. In recognition of this uncertainty it has become customary to refer to newly discovered bodies of this sort as "virus-llke" particles. Actually, little is gained in accuracy by saying that they are "like" other particles found in virus infected cells when the viral nature of those other particles has not been definitely established. "Particles-presumed-to-be-virus" would be a more conservative term but this expression is all too cumbersome to use repeatedly. We have decided therefore, in the interests of simplicity and readability to omit the qualifying phrases and to refer simply to the "virus particles." It is hoped that the reader will understand that we hold the same reservations about their identification as others who use the term "virus-like particles."
Fawcett went on to state that although the electron micrographs provided the first visual evidence of particles in the tumor cells that might be the postulated tumor agent, the nature of these particles remained uncertain. The “virus” claim was based upon the resemblance to what had been previously deemed herpes “virus” particles. While noting that they could also be from an unrelated “virus,” Fawcett acknowledged that the particles were not present in all tumors or in all cells, adding to the uncertainty of their “viral” nature. Furthermore, he noted that there were two types of tumors observed: those with conspicuous nuclear inclusions, where “virus-like” particles were evident, and those without.
“The electron micrographs obtained in the present study provide the first visual demonstration of particles in the tumor cells that might be the postulated tumor agent. Because of their great morphological similarity to the particles observed by Morgan et al. in cells experimentally infected with herpes simplex and other viruses, it is easy to conclude that the particles found in the Lucke tumor are virus particles of some kind. The possibility cannot be excluded, however, that they may be a virus that is unrelated to the causation of the tumors. The characteristic particles are not found in all of the tumors examined and when they are present they are not seen in all of the cells. When studied in histological sections, the kidney tumors fall into two categories, those in which conspicuous nuclear inclusions are present and those in which they are lacking. Our experience to date seems to warrant the generalization that when typical inclusions are seen in a tumor with the light microscope, virus particles will be found in electron micrographs of the same tumor. In the absence of nuclear inclusions the particles are either entirely lacking or they are present in such small numbers as to escape detection by the inadequate tissue sampling afforded by electron microscopy.”
Fawcett, recognizing the limitation of static electron microscopic images, remained cautious in his interpretation, noting that these images could not definitively reveal the dynamics of the hypothetical “viral” process in living systems. In other words, static images of extensively altered, deceased material cannot provide any insight into the processes that occur in a living system in its natural state.
“Although fully cognizant of the uncertainties involved in any effort to reconstruct a dynamic process from a limited number of static images, we submit, in resume, the following tentative interpretation of the intracellular formation of virus particles in the Lucke tumor.”
Fawcett emphasized that he had no definitive proof that the particles he imaged were “viruses” capable of causing tumors. Nonetheless, he considered it plausible that they could represent the elusive invisible agent. He also acknowledged that, since two-thirds of the tumors showed no inclusion bodies or “virus-like” particles, his hypothesis would require the rescue device concept of “latent viruses” to explain the contradictory evidence. In the end, Fawcett left open the possibility that, while these particles may or may not be “viruses,” there was no conclusive evidence that they were causally linked to tumor formation.
“There is as yet no definitive proof that the virus particles described here are the causative agent of the tumors but it does not seem unreasonable to believe that they may be. If this assumption is made, the problem of masked or latent virus immediately arises out of the necessity for explaining the fact that nearly two-thirds of the tumors show no inclusion bodies with the light microscope and no recognizable virus in electron micrographs. Are the cells of such tumors actually free of virus or is it present in a latent form that at present cannot be identified morphologically?”
“Approximately a third of the tumors examined were found to contain spheroidal bodies of uniform size and distinctive morphology that are believed to be virus particles. These consist of hollow spheres (90 to 100 mu) having a thick capsule and a dense inner body (35 to 40 mu) that is eccentrically placed within the central cavity (70 to 80 mu). Virus particles of this kind occur principally in the cytoplasm but occasionally they are also found in the nucleus and in the extracellular spaces of the tumor.”
“These findings are discussed in relation to previous work suggesting that the Lucke adenocarcinoma is caused by an organ-specific filtrable agent. It is concluded that the "virus particles" found in electron micrographs of the tumor cells may be the postulated tumor agent. On the other hand, the possibility remains that the particles described here are not those that are causally related to the tumors.”
Fawcett's paper ended with various images from his electron microscopy study of “virus-like” particles. Provided are a few examples with the descriptions. Do you see a cluster of “mature virus particles,” or is this simply another case of point-and-declare at the particles that fit the preconceived idea of what a “virus” is supposed to look like?
https://pmc.ncbi.nlm.nih.gov/articles/PMC9041479/
In 1957, Adams and Prince conducted electron microscope studies on Ehrlich ascites tumor (EAT) cells, identifying distinctive particles closely associated with the endoplasmic reticulum of normal (or “uninfected”) cells. Their findings were corroborated by other researchers, confirming that these particles were not exclusive to “virus-infected” cells as previously believed.
Since the morphology of these particles resembled what had been interpreted as “viruses,” the researchers systematically examined different sublines of Ehrlich ascites tumors to determine whether the particles were characteristic of EAT cells or appeared only sporadically. They considered this investigation crucial, especially after Selby et al. had reported a different type of intracellular “virus-like” particle in “uninfected” EAT cells, which they suspected might indicate contamination with an unknown “virus.”
An electron microscope study of the morphology and distribution of the intracytoplasmic virus-like particles of Ehrlich ascites tumor cells
“During the course of a series of electron microscope studies on Ehrlich ascites tumor (EAT) cells, carried out in this laboratory, a distinctive particle was identified in close association with the endoplasmic reticulum of normal cells. Coincident with our findings this same observation was made by Caulfield and Porter (cited in reference 4) and by Moore (1). Similar particles had previously been reported in EAT cells infected with Anopkdes A virus and had been tentatively identified as the intracellular form of this virus by Friediaender et al. (2, 3). As the studies described in the present paper neared completion a report by Friedlaender and Moore (4) appeared describing the occurrence of these particles in the endoplasmic reticulum of uninfected EAT cells.
Since the morphology of these particles suggested that they might represent virus, it seemed of some importance to conduct a systematic examination of several different sublines of the Ehrlich ascites tumor in order to determine whether they were truly characteristic of the EAT cell, or whether they occurred only sporadically. The necessity for carrying out extensive studies of many different sublines of tumor obtained from different geographic locations in order to establish this point is emphasized by the rare finding of a different type of intracellular "virus-like" particles in uninfected Ehriich ascites tumor cells by Selby et al. (5), which was thought by these investigators to be due to contamination with unknown virus.”
The researchers acknowledged that while the particles could hypothetically be a “virus,” their actual nature remained unclear. If they were a “virus,” it would imply that the Ehrlich ascites tumor was an obligate carrier of this “virus.” However, the only basis for considering them “viral” was morphological resemblance to other particles previously assumed to be “viruses,” rather than any clear biological function.
Doubt was cast on the “viral” hypothesis due to repeated failures to isolate an “infectious agent” from EAT cells. This raised the possibility that the particles were either an unrecognized normal cytoplasmic component or a byproduct of cellular processes such as phagocytosis—potentially remnants of cellular digestion rather than evidence of “viral infection.”
“Although the nature of these particles is not clear, two possibilities exist. One possibility that must be considered is that they do actually represent virus. If this is the case their occurrence in several different tumor cell sublines propagated in different strains of host mice and obtained from different geographic locations would, as noted above, argue strongly against inadvertent laboratory or mouse sources of viral contamination. This consideration would tend to implicate the Ehrlich ascites tumor as an obligate carrier of the postulated virus. At present the only evidence indicating that this particle might be virus is its morphology, which is more closely akin to that which has been demonstrated for some viruses than to that of any normal cellular component which has yet been described. The Ehrlich ascites tumor is thought to have arisen from the mouse mammary tumor (16) (although documentary proof of this origin has been lost), and in this vein it is interesting to note that Bernhard (17), in a study of the mouse mammary tumor, has demonstrated intracellular particles identified as the mammary tumor agent which bear some resemblance to the spherical (i.e. not attached to the surrounding vesicle) forms of the above described particles. To complete this comparison, however, it should be pointed out that the intracellular particles which Bernhard found were not associated with the endoplasmic reticulum. Further doubt is cast on the suggestion that the particles might represent virus by the failure of attempts to isolate an etiological agent from EAT cells (4, 18).
A second possibility, of course, is that these structures represent a heretofore unrecognized "normal" cytoplasmic component. This idea receives token support from the fact that although vesicle formation has been reported (7) and virus-like particles have occasionally been demonstrated within membranes thought to be endoplasmic reticulum (19), none of several recent electron microscope studies of intracellular virus (7, 19-28) has demonstrated such a constant and obvious association of particles with either the rough surfaced or smooth surfaced portions of the endoplasmic reticulum. Countering this is a rapidly increasing mass of morphological data covering scores of cell types which has so far not demonstrated similar particles in cells other than those of the Ehrlich ascites tumor.
A third and much less likely possibility is that the particles represent some form of or product of phagocytosis.”
After extensive study, the authors concluded that the particles were intrinsic components of Ehrlich ascites tumor cells and probably did not represent a contaminating “virus.”
“It is concluded that these structures are a constant morphological component of the Ehrlich ascites tumor and that they probably do not represent contaminating virus. Their morphology and distribution are described, and the possible interpretations of their significance are discussed.”
These are the published images of the “virus-like” particles found in the electron microscopy examination of “uninfected” Ehrlich ascites tumors that were concluded to most likely be either an unrecognized normal cytoplasmic component or a byproduct of cellular processes.
https://pmc.ncbi.nlm.nih.gov/articles/PMC2224075/
Both of the studies from the 1950s highlight the uncertainty surrounding electron microscope observations of “virus-like” particles due to the morphological resemblance to previous particles assumed to be “viruses. They underscore the importance and necessity of being able to distinguish between normal cellular structures, cellular debris, artifacts, and hypothetical “viral agents,” as well as the need for clear scientific evidence to demonstrate that the observed particles are, in fact, “pathogenic viruses.”
In the 1960s, the use of electron microscopy continued to grow in identifying “virus-like” particles in tissue and cell culture samples. It was during this decade that June Almeida first identified the “coronavirus” in 1967 from unpurified samples taken from children with a “flu-like” illness. As had been done in earlier research, “virus-like” particles observed through EM, resembling previously described animal “viruses,” led Almeida to conclude that a new family of “viruses” had been discovered in humans. Interestingly, these images were initially dismissed as poor representations of influenza particles.
Identification of a New Type of Virus
“Tyrrell’s team of researchers had collected samples of a flu-like virus from a sick schoolboy. However, they were having trouble cultivating this virus using traditional methods, leading them to suspect that this might be a completely new type of virus (Broklehurst 2020).
Tyrrell sent some of these samples to Almeida in the hopes that she would be able to identify the virus using the IEM technique. Almeida was able to successfully produce clear images of the virus and noted that it resembled two similar viruses she had previously imaged while studying bronchitis in chickens and murine hepatitis (Brocklehurst 2020). These had previously been dismissed as poor images of influenza viruses. However, the images of the virus in the samples from the schoolboy combined with the images from Almeida’s previous work convinced Almeida and Tyrrell that these were in fact a new family of viruses.
https://www.bio-rad-antibodies.com/blog/the-remarkable-story-of-June-Almeida.html
As cell cultures became a more prominent tool after John Franklin Enders introduced the technique in 1954, virologists began finding these same “virus-like” particles in tissues with no known “viral” cause, as well as in “uninfected” cells. Even though these particles were morphologically indistinguishable from those thought to be “viruses,” researchers could not demonstrate any biological or “viral” activity.
For example, in 1961, Dales and Howatson conducted electron microscope studies on three sublines of Earle's L strain cells. They observed “type C virus-like” particles in two of the three sublines, both within cytoplasmic vacuoles and outside the cells near the cell surface. These particles appeared to form through a budding process at the cell membrane. Similar particles were found in the culture medium and in tumors induced in C3H mice by inoculating them with the cultured cells. Additionally, a second type of particle, termed “type A virus-like,” was present within cytoplasmic vacuoles in all three sublines. However, no biological activity had been associated with either type of particle.
Virus-like Particles in Association with L Strain Cells
Summary
Electron microscope studies of three sublines of Earle's L strain cells showed that in two of the three sublines virus-like particles of type C were present within cytoplasmic vacuoles and outside the cells close to the cell surface. The particles formed by a budding process at the ceil membrane. Similar particles were observed in pellets prepared from the medium in which the cells had been grown, and in cells of tumors produced in C3H mice by inoculation of the cultured cells. A second type of particle, type A, was observed within cytoplasmic vacuoles in all three sublines of cells cultivated in vitro. No biological activity has so far been associated with either type of particle.
The researchers noted that, with the advent of tissue and cell culture techniques, significant efforts had been made to identify structures resembling “viruses” within various tumor cells. While such particles were frequently observed, definitive “viral” identification was rare, as most lacked biological evidence of “viral” origin. Consequently, the significance of these “virus-like” particles remained uncertain without demonstrable biological activity.
“Since methods for preparing cells and tissues for thin sectioning and electron microscopy have become available, considerable effort has been expended in searching for structures that might be identified as viruses within and around cells of many different types of tumors. In tumors of known viral etiology these efforts have been for the most part successful in that particles presumed to be the causative agents have been observed in many instances (3), although it should be noted that in only a very small number of these has the further step required for unequivocal identification been made, namely, the establishment of a correlation between the number of particles of the type observed and tumor-inducing activity (25). In a number of tumors for which there is no biological evidence of viral origin, structures morphologically similar or identical to those observed in certain virus-induced tumors have been described (3). The significance of such "virus-like" particles is difficult to assess in the absence of any demonstrable biological activity. Nevertheless, it cannot be assumed that their presence in and around tumor cells is fortuitous, and it is of some importance to determine their relation to the tumor cells and frequency of occurrence in different tumors. This is especially true in view of current investigations of the possible viral etiology of human tumors in which morphological observations may be important, since many experimental approaches used with animals are not feasible in human beings.”
As stated in the opening summary, the electron microscope studies were performed on three different sublines of the L strain, on tumors from the injection of cells from the sublines into newborn C3H mice, and on pellets from the cell culture media.
Specimen material.—Electron microscope studies were made on three types of specimen: (a) cells of each of the three sublines cultivated m vitro, (b) tumors that resulted from giving injections to newborn C3H mice of cells of the three sublines, and (c) pellets prepared from the nutrient media in which the cells had been grown.
Dales and Howatson discussed the possibility that the “type C virus-like” particles might be the agents responsible for the original transformation of cells to malignancy. However, they deemed that this seemed unlikely, especially since one subline did not contain these particles. They also considered that these particles might represent tumor “viruses,” noting instances where “viruses” unrelated to the causative agent were recovered from tumor tissues. The researchers felt that from a purely morphological standpoint, the “virus-like” particles were indistinguishable from “known” tumor “viruses” both in structure and in mode of formation, but the significance of such morphological evidence was open to question in the absence of demonstrable biological activity.
Dales and Howatson had actually attempted to demonstrate “viral” activity by injecting pellets of the cultures subcutaneously into newborn CSH mice and Syrian hamsters. However, after 8 months, no biological activity of the preparations was detected. Amazingly, the researchers attempted to downplay the negative results, saying that they do not rule out the possibility that the “virus-like” particles are tumor “viruses” as the right conditions for tumor formation may not have been generated.
“We shall consider first the type C particles. These particles have the general morphological characteristics that are associated with many known viruses including several tumor viruses, namely, a dense central body or nucleoid surrounded by a less dense zone, the whole being enveloped in a dense limiting membrane. It is possible that they represent the etiological agent that was responsible for the original conversion of the cells to malignancy, but there is no evidence in support of this; and it would seem unlikely, since one subline did not contain any particles. Nevertheless the particles may represent tumor virus. There are now several instances of the recovery from tumor tissue of tumor virus of a type apparently unrelated to the agent causing the tumor from which it was recovered. For example, polyoma virus has been recovered from leukemic tissue (23) and from mammary tumor (16) of the mouse in the induction of which other viruses, both distinct from polyoma, are implicated. Again, a virus causing leukemia has been obtained from a sarcoma for which there is no evidence of viral origin (17). Thus it would seem that tumor viruses may be carried as passenger viruses and that tumor cells provide a suitable environment for them. Further, the mode of formation of the particles that we observed around L cells, namely, a budding process at the cell membrane, closely resembles that described for certain tumor viruses, the identity of which is reasonably well established (3). The most detailed studies have been made by deHarven (10) on the leukemia virus of Friend. Thus, from a purely morphological standpoint, the VL particles are indistinguishable from known tumor viruses both in structure and in mode of formation. However, as already pointed out, the significance of such morphological evidence is open to question in the absence of demonstrable biological activity.
To test whether the particles released from the two strains of L cells had any biological, particularly tumor-inducing activity, pellets of the particles obtained as described were resuspended in buffered saline solution and injected subcutaneously into newborn CSH mice and Syrian hamsters. To date, 8 months later, no biological activity of these preparations has been detected. These negative results do not, of course, rule out the possibility that the VL particles are in fact tumor viruses, for it is known that the action of some tumor viruses is strongly conditioned by host factors, and the conditions required to demonstrate biological activity may not have been satisfied in the limited tests that we have carried out.”
The study also suggested that these “virus-like” particles might not be “infectious units” but could result from a secretory process within the cells, implying they are normal cellular components rather than “pathogenic viruses.” This was supported by the observation that particle production did not affect cell growth or health and was consistent across different environments, indicating that the “virus-like” particles may be an endogenous (internal) part of the cell's normal processes rather than a result of an exogenous (external) “viral infection.”
“A remaining possibility is that the particles are not infectious units but are a product of some kind of secretory process in the cells. This presumed secretory activity did not seem to disturb cellular function, for the lines of cells that produced particles grew as rapidly and appeared as healthy as the one that did not. Further, particle production was not influenced by environmental factors, since the two lines that produced particles did so whether grown in vitro or in vivo.”
Regarding the “type A virus-like” particles, their “viral” nature was also uncertain. These particles were not found in the culture medium which prevented biological activity testing. Their classification as potential “viral” agents was based solely on morphological grounds. While the particles were found in tumors of “known viral etiology,” the same particles were observed in tumors where “viral” etiology had not been demonstrated. The researchers proposed that these particles might be a cellular response to the presence of a “virus” or another abnormal condition, or possibly an abnormal type of secretion.
“The viral nature of the type A particles is also questionable. It appears that they are not released from the cells as are the type C particles, because they were not present in the pellets prepared from the nutrient medium in which the cells were grown. It was therefore not possible to test the type A particles for biological activity in the same way as the type C particles. Their claim to be considered as possible viral agents rests on purely morphological grounds. Particles of type A have been described in cells of many different types of tumor in mice. They may occur sparsely or in large aggregates in tumors of known viral etiology such as mouse mammary tumors (4, 5), or they may be present in tumors for which viral etiology has not been demonstrated (15). It has been suggested that they represent a stage in the formation of mature virus particles (3), but they may equally well be a reaction of the cell to the presence of virus or to some other abnormal condition. Although in some tumors a close association has been observed between the Golgi region, known to be concerned in secretory activities of the cell, and type A particles (5, 15), it is not evident in L cells. Nevertheless, the possibility that type A particles in L cells represent some abnormal type of secretion should not be overlooked. Type A particles seem to be associated with neoplasia, for which they have not to our knowledge been described in non-neoplastic cells; but their role and significance, if any, in the neoplastic process remains to be determined.
In conclusion, Dales and Howatson highlighted the prevalence of “virus-like” particles in various tumors without demonstrated “viral” activity. They emphasized the need for additional evidence before attributing a role in carcinogenesis to these particles based solely on their resemblance to “known viruses.” Even when isolating these particles in “relatively pure” forms, obtaining evidence of their biological activity remained challenging.
Conclusion
There is now a long list of tumors both in animals and in human beings in association with which particles suspected of being virus have been observed, but for which no viral activity has been demonstrated biologically. These include Ehrlich ascites tumor cells (1, 21), plasma-cell tumors (15), human leukemic lymph nodes (7), and mammary tumor (14), and uterine epithelioma (13) in the rat. Whereas such observations are of interest and may be of great significance, it is clear that more evidence must be acquired before any claim to a role in carcinogenesis can be attributed to particles because of their resemblance to known viruses. The present studies show that, even when it is possible to isolate the "virus-like" particles in fair quantity and in fairly pure state, it may still be difficult to obtain evidence that they have any biological activity.
Seven years later, Cromack reported the presence of “virus-like” particles in chick embryo and L cell cultures through electron microscopy. She noted that many researchers had observed similar particles in normal—i.e., “uninfected”—chicken cell cultures and speculated that they could be “latent viruses” associated with avian lymphomatosis, a condition often present without causing disease. In her study, Cromack observed these particles in “uninfected” chicken embryo cultures and found that those incubated for four days before fixation contained significantly more particles than those fixed after one day, despite the absence of cytopathic effects.
An Electron Microscope Study of Virus-like Particles in Chick Embryo and L Cell Cultures
“Latent virus infections in chickens have been recognized for several years. Many investigators have seen spherical, virus-like particles in normal chicken cell cultures, and have suggested that they might have been the virus of avian lymphomatosis, which occurs frequently without producing disease symptoms and is transmitted trans-ovarially.
Virus-like particles were also observed during the present study of the ultrastructure of primary monolayers from 10-day-old chick embryos cultured in Eagle's (I955) medium supplemented with 3 % calf serum. Cultures were prepared according to the method of Dulbecco & Vogt (I954) from a Heisdorf and Nelson hybrid White Leghorn commercial breed of chickens; 56% of the cells had virus-like particles either at their periphery or internally. P1. I, fig. I shows a group of extracellular particles in a chick embryo culture incubated for 4 days; their mean diameter was 1060 A (Table I). Each particle consisted of a dense central nucleoid surrounded by a membrane, then a clear area finally bounded by an outer membrane. Both the chicken and murine virus-like particles (discussed later) were fixed with either OsO 4 or glutaraldehyde followed by OsO4 post-fixation, and then studied with a Siemens Elmiskop I electron microscope. Sections were cut on a Servall MTI Porter-Blum ultramicrotome. The particles were almost exclusively extracellular (P1. I, fig. 2). Occasionally they were seen in cytoplasmic vacuoles near the cell surface, and, rarely, in cytoplasmic vacuoles near the nucleus (P1. I, fig. 3). When chick embryo cultures were incubated for 4 days before fixation instead of one, the particles present appeared to have increased greatly in number, although cytopathic effects did not occur.”
Cromack noted that Febvre & Benedetti (1958) had observed similar “virus-like” particles in “uninfected” White Leghorn cells, which exhibited no cytopathogenic effects, just as in her own study. She also referenced earlier research by Dales and Howatson (1961) and Kindig and Kirsten (1967), which described the same phenomena. Despite the frequent presence of these particles in cell cultures, Cromack acknowledged that no biological activity had been demonstrated, reinforcing the fact that “virus-like” particles can exist in cell cultures without a clear biological function or evidence of “infectiousness.” The fact that they were already present in cells prior to the supposed “infection” with “Western equine encephalitis (WEE) virus” suggests that these particles are intrinsic to the cultured cells rather than the result of “viral infection.” This challenges the assumption that all observed particles in virology experiments are necessarily “pathogenic viruses.”
“Febvre & Benedetti (1958) examined normal White Leghorn, as well as Brown Leghorn, cells and saw virus-like particles only in the former; their Brown Leghorns were supposed to be free from avian lymphomatosis virus. Their particles were similar in morphology to those described here, most of the particles were extracellular, and no cytopathic effects were observed.
In the second part of this study, a morphologically different type of virus-like particle was seen in two lines of cultured strain L 929 mouse cells. Lockart (I963) has described culture methods for the cells. The particles are identical to those observed by Dales & Howatson (1961) in strain L mouse cells. Kindig & Kirsten (1967) also reported seeing them after the present work had been completed. One type of routine particle, resembling a spherical "doughnut', is found both within and outside the cell. According to Bernhard's & Gurrin's (I 958) morphological classification of murine leukaemia particles, it is of type A. Dales & Howatson (1961) also observed type A virus-like particles in 10 to 20% of their cells. Intracellular and extracellular particles of this type described here consisted of an electron transparent nucleoid and a dense outer area (P1. 2, fig. 5). In addition, the extracellular type A particles possessed two concentric membranes outside the dense area, and sometimes contained a nucleoid of intermediate density. Intracellular particles averaged 810 A in diameter, while extracellular ones averaged 1030 A. The smaller particles budding from the cell membrane in PI. 2, fig. 5 are WEE virus. Clusters of immature WEE viruses free in the cytoplasm, and ribosome clusters associated with WEE virus development, also were observed in other cells. The electron microscopic observation of WEE virus multiplying to high titres in L cells containing virus-like particles has not been previously reported.
The other type of murine particle seen rarely in L cells was of type C, according to Bernhard's & Guerin's (1958) classification. Similar particles were reported by Dales & Howatson (I960), but they could not determine any biological activity for either type of particle. The spherical type C particles averaged 1000 A, in diameter and contained a dense central nucleoid and an outer region of intermediate density surrounded by a membrane (P1. 2, fig. 8).
Cromack reported that none of the 36 samples from L cells in continuous subculture over a 2-year period was found to be devoid of “type A virus-like” particles, with 91% containing the entities. This finding reinforced that these structures are intrinsic to L cells in culture, rather than being externally introduced “infectious agents.”
She noted that these particles resembled those observed in mice with leukemia and in diseases of uncertain “viral” origin, such as plasma cell tumors, renal carcinomas, and Ehrlich ascites tumors. However, despite clear evidence that these particles were naturally occurring in L cells and the absence of any demonstrated biological activity—meaning no proof of “infectiousness” or disease causation—Cromack ultimately classified them as “viruses” based solely on their morphological appearance in electron microscopy.
“None of 36 samples from L cells in continuous subculture over a 2-year period was found to be devoid of type A virus-like particles; where counts were made, 91% of the cells contained these entities. These particles are similar in morphology, size and location in the cell to viruses found in mice having leukaemia, a disease of known viral aetiology (de Harven & Friend, I958). They also strongly resemble those seen in diseases of uncertain viral aetiology, such as the development of plasma cell tumours (Parsons et al. I96I), renal carcinomas (Claude, I962), or Ehrlich ascites tumours (Adams & Prince, I957).
The viral nature of the chicken and murine virus-like particles is strongly suggested by their small dimensions, uniform size, and observation of them with the electron microscope in preparations fixed by several methods. Although biological activity has not been established, it is believed on morphological grounds that both types of particles are respectively chicken and murine oncogenic viruses.”
https://pubmed.ncbi.nlm.nih.gov/5645579/
The flawed reasoning displayed by Cromack is a perfect example of how “virus-like” particles in cell cultures are often assumed to be “pathogenic viruses” without direct evidence of “infectivity” or disease causation. Coupled with the work of Dales and Howatson, it demonstrates a fundamental weakness in the logic of identifying “viruses” purely through electron microscopy.
As researchers examined more substances under electron microscopy, they increasingly discovered morphologically identical “virus-like” particles in unexpected places. In 1970, Breese Jr. reported observing such particles, identical in morphology to “virus” particles found in baby hamster kidney (BHK-21) cell cultures and mouse leukemia, in two stable pig kidney cell lines, PK-131 and IB-RS-2.
Electron microscopy was used to examine tissue cultures of both cell lines that were “infected” with “African swine fever virus” (ASFV) or “foot-and-mouth disease virus” (FMDV), alongside “uninfected” controls. While “virus-like” particles were observed in the “infected” cultures, identical particles were also present in the “uninfected” cultures as well. Notably, the IB-RS-2 cell line—untouched by “infection” or passage since its arrival at the laboratory—contained these particles in every sample examined, whether “uninfected” or “infected” with another “virus.”
Virus-like Particles Occurring in Cultures of Stable Pig Kidney Cell Lines
“Several reports in the literature (1--3) have dealt with virus particles which have been seen in baby hamster kidney (BHK)-21, clone 13 cell line cultures (4). Other investigators (5) have seen morphologically similar particles in BHK cells when they have been infected with a number of viruses such as Bunyamwera, Rubella, St. Louis encephalitis, and Eastern equine encephalitis. More recently, virus-like particles have also been shown in a bovine cell line, designated CK-66, developed at the Federal Research Institute for Animal Diseases, Tfibingen, Germany (6). The reports generally indicate that the number of virus-like particles increases when the cell cultures are infected with the test virus. This note reports the occurrence of particles, which are identical in morphology to those referred to above, in two stable pig kidney cell lines, PK-131 and IB-RS-2 (7).
Tissue cultures of the two cell lines in 4-oz. prescription bottles, both infected with African swine fever virus (ASFV) (8) or foot-and-mouth disease virus (FMDV) (9, 10) and uninfected controls, were prepared for electron microscopy.”
“Figures 1 and 2 show typical examples of the virus-like particles in sections of PK-13 and IB-I~S-2 cells which had been infected with ASFV and FMDV, respectively. Identical particles were found in uninfected cells, but the occurrence was less frequent and they were not always seen from passage to passage. Developmental stages have rarely been seen and only occasionally do the virus-like particles seem to be budding from the cell membrane. The particle diameter is approximately 90 mu and the core is c. 60 mu. The illustrative micrographs were chosen from many which have been taken in the course of other investigations. The appearance of the virus-like particles in PK-13 cells had been observed for several years. The IB-RS-2 cell lines, which had not been infected or passaged since arriving in our laboratory, was also examined for the virus-like particles. They were present in every cell sample whether uninfected or infected with another virus. Passages of fluids of cells showing particles into primary swine kidney tissue cultures and subsequent electron microscopic study revealed no particles.”
https://pubmed.ncbi.nlm.nih.gov/4195629/
This study demonstrated that identical particles appeared in both “infected” and “uninfected” cells, challenging the assumption that their presence indicated “viral infection.” If these particles were truly “viral,” they should not be found in the “uninfected” samples. The inconsistent appearance, lack of clear replication stages, and failure to propagate in new cultures suggests that the particles arre more likely cellular structures, artifacts of electron microscopy, or other “non-viral” substances rather than “pathogens.”
Breese Jr.’s report was already a significant blow to the “virus” narrative at the start of the decade, but an even more striking discovery emerged in the mid-1970s: the same “virus-like” particles were found in “virus-free” fetal bovine serum (FBS)—the very medium used to maintain cells and culture “viruses.” In 1974, Benz Jr. and Moses examined twenty-two samples of bovine sera and found the presence of “virus-like” particles in all tested bovine serum samples, including those labeled as “virus-screened” fetal bovine serum and agamma calf serum. The fact that these structures were found universally, even in “virus-free” samples, raises serious questions about their true nature. The researchers used ultracentrifugation and electron microscopy to detect these particles, finding them in fractions with a density of 1.16-1.18 g/cc when subjected to a cesium chloride (CsCl) gradient. Notably, these densities overlap with those often attributed to “viral” particles in virology studies.
Brief Communication: Small, Virus-Like Particles Detected in Bovine Sera by Electron Microscopy
SUMMARY-Twenty-two samples of bovine sera were examined by ultracentrifugation and electron microscopy for the presence of virus-like structures. All samples, including those of “virus-screened" fetal bovine serum (FBS) and agamma calf serum, had such structures. When pellets of FBS were resuspended in buffer and spun to equilibrium on a continuous 1.1-1.3 glcc CsCI gradient, virus-like particles identical to those seen in the FBS pellets were observed in fractions with a density of 1.16-1.18 g/cc. In samples of FBS used to support the growth of 2 human Iymphoblastoid cell lines from patients with acute infectious mononucleosis, peak incorporation of radioactive uridine was observed in the 1.17-1.18 g/cc densities; fractions with the highest radioactivity also contained the most small, virus-like particles.-J Natl Cancer Inst 52: 1931-1934, 1974.
The researchers noted that while cell culture techniques increase the probability of detecting “agents” produced by tumor cells, they also increase the likelihood of introducing “adventitious agents” that may or may not be detected. They acknowledged that bovine sera are frequently contaminated with “viruses” and that even suppliers claiming to screen for these “viruses” may still provide sera containing unidentified “virus-like agents.” They warned that contamination of these lines with bovine “viruses or virus-like agents” may obscure the presence of the candidate tumor “virus” or may itself be mistaken for a candidate human tumor “virus.” In other words, researchers might misidentify these foreign “virus-like” particles as human oncogenic “viruses,” raising concerns about the validity of studies that claim to identify “new” tumor-associated “viruses,” as they could simply be detecting “adventitious agents” from culture conditions rather than human “pathogens.”
THE SEARCH for oncogenic viruses in human tumors has led investigators to use in vitro techniques to augment the sensitivity of existing methods of virus detection (1, 2). Many of these involve cultivating tumor cells through several passages in tissue culture. Cell culture techniques, while increasing the probability of detecting agents produced by tumor cells, increase the probability of introducing adventitious agents that may or may not be detected.
Sera of bovine origin are frequently contaminated with bovine viral diarrhea virus (BVD), infectious bovine rhinotracheitis virus (IBR), and parainfluenza 3 (PI-3) (3, 4). Some commercial suppliers screen for these specific viruses and offer bovine sera presumably free of these agents. At least one supplier offers fetal calf serum screened for viruses or virus-like agents not otherwise detectable during screening for BVD, IBR, and PI-3. Since many tumor cell lines are established and carried with sera of bovine origin, contamination of these lines with bovine viruses or virus-like agents may obscure the presence of the candidate tumor virus or may itself be mistaken for a candidate human tumor virus.”
In the next section, Benz Jr. and Moses report that all fetal bovine samples they examined, including “virus-free” ones, contained numerous “virus-like” particles. These same particles were noted in lesser quantities in calf and adult bovine serum, and lamb and porcine serum contained scattered similar particles. Interestingly, horse and human serum contained no detectable “virus-like” particles. This could explain why virologists often prefer fetal bovine serum over human serum for their cell culture studies.
Results and Discussion
Twenty-two sera of 5 species obtained from 5 commercial suppliers were examined by ultracentrifugation and electron microscopy for the presence of virus-like structures. All samples of bovine sera examined to date, including those of "virus-screened" FBS and agamma calf serum, had such structures. These particles were numerous in all 10 batches of FBS examined and were noted in lesser quantities in calf and adult bovine serum. Lamb and porcine serum contained scattered similar particles, whereas horse and human serum contained no detectable virus-like structures (table I). These small, virus-like particles consisted of a central core of varying density surrounded by a membrane envelope and had a median diameter of about 55 nm (fig. 1).
When pellets of FBS were resuspended in TKM buffer and spun to equilibrium on a continuous 1.1-1.3 gl cc CsCl gradien t, virus-like particles identical to those seen in pellets of FBS were observed by electron microscopy in fractions with a density of 1.16-1.18 glcc (fig. 2).”
The researchers emphasized the potential for contamination of bovine serum with “virus-like” particles that could interfere with the detection and study of human tumor “viruses.” This represents a significant issue for researchers growing human tumor lines, as any contamination from supposed bovine “viruses” could skew the results. The “virus-like” particles detected in the bovine serum were found to band in the same region as RNA tumor “viruses,” which raises the risk of misidentifying these particles as tumor “viruses.” Benz Jr. and Moses concluded that FBS is widely contaminated with “virus-like” particles of unknown biological properties, and that such contaminants introduce an uncontrolled variable into studies attempting to detect and propagate a human tumor “virus,” whose properties remain equally unknown.
“The presence of virus-like structures in all samples of bovine serum examined in this laboratory represents a serious potential obstacle to the investigator screening human tumor lines grown in bovine sera for the production of a human tumor virus. 1) The presence of a bovine virus contaminant could interfere directly with the establishment of human tumor lines or with tumor virus production. FBS has been reported to inhibit spontaneous neoplastic transformation of mouse cells in vitro (6). 2) A serum virus contaminant could interfere with the detection of endogenous tumor virus production, since the small, virus-like particles seen in this laboratory band in the equilibrium density region where RNA tumor viruses would be expected to band (7). The 3H-uridine incorporation data suggest that the particles may be biologically active; however, the exclusive detection of these structures in the fractions with greatest incorporation of 3H-uridine does not necessarily mean that these agents are responsible for uptake of the isotope. If the particles are biologically active, the detection of an RNA tumor virus from a tumor cell line grown in FBS, by incorporation of 3H-uridine followed by equilibrium density centrifugation, would be equivocal, especially if little endogenous agent is produced. At least these findings suggest that FBS is widely contaminated with virus-like particles of unknown biologic properties. The possibility of such a contaminant adds an uncontrolled variable to studies attempting to detect and propagate a human tumor virus whose properties are likewise unknown.”
https://pubmed.ncbi.nlm.nih.gov/4834422/
Similar results to Benz Jr. and Moses was obtained two years later in 1976, when Calafat et al. reported the presence of “virus-like” particles in nine different commercial bovine sera used for tissue culture. These particles were spherical, with diameters ranging from 70 to 95 nm, and had surface projections measuring approximately 11 to 12 nm. Despite their similarity to “known viruses,” only one of the nine samples tested positive for “Bovine viral diarrhea virus” (BVDV) using an Ouchterlony test. The researchers emphasized the importance of ensuring that “virus-like” particles are not present in tissue culture media, as their presence can complicate studies on “virus” production.
Virus-like Particles in Bovine Sera for Tissue Culture
Summary
“Virus-like particles were found in nine different bovine sera for tissue culture from commercial suppliers. These particles were spherical with an overall diam. between 70 and 95 nm. After negative staining, surface projections of about 11 to 12 nm were clearly seen. One of the nine sera was positive in an Ouchterlony test with antisera against bovine viral diarrhoea virus.
In many studies on virus production in tissue culture it is important to search for virus particles in the medium (Todaro, Zeve & Aaronson, I970). In cases where the virus production of the cells is very low or the morphology of the virus particles is not well established it is necessary that the tissue culture medium itself, especially the serum component, does not contain virus-like particles.
During their investigation, the researchers initially detected “virus-like” particles in the medium of monolayers producing so-called “bovine leukemia virus” (BLV). Alongside these, they observed smaller but morphologically similar particles (~80 nm) with surface projections. Since these smaller particles were absent in thin sections of the cell cultures, the researchers turned their attention to the calf serum itself. Upon examination, every sample of bovine serum contained abundant “virus-like” particles with surface projections of about 11 to 12 nm.
The researchers noted that other “viruses,” such as “bovine syncytial virus” (BSV), exhibited a similar morphology and surface projections of comparable length. “BVDV” was found to have a similar diameter to these particles, but at the time, it was unclear whether it possessed surface projections. Later studies “confirmed” that “BVDV” does, in fact, have such projections.
To determine whether the observed particles were actual “viruses,” the researchers conducted serological tests against “BVDV,” “parainfluenza-3 (PI-3),” “infectious bovine rhinotracheitis virus (IBRV),” “BSV,” and “BLV.” Only the sample from Bio-cult Laboratories reacted with “anti-BVDV” serum, while the others yielded negative results. Thus, the testing did not determine that the morphologically identical “virus-like” particles were a “virus.”
Given that “virus-like” particles were detected in all tested serum samples, the study raised concerns about their potential to interfere with morphological studies of “viruses” in tissue culture. The researchers concluded that these particles were most likely contaminants from fetal bovine serum rather than genuine “viral isolates.” They emphasized that without additional verification, “virus-like” particles cannot be identified as “viruses” based on morphology alone.
In a previous report (Calafat, Hageman & Ressang, I974) we described the morphology of bovine leukaemia virus (BLV) in short-term cultures of leukocytes from cows with persistent lymphocytosis. These particles were found in thin sections of the cells and after negative staining of material concentrated from cell culture medium. The yield of virus-like particles from the cells culture fluid was very low but after negative staining, particles could nevertheless be recognized on account of their clear surface projections. Thin sections from the same pellet showed also BLV particles. The diam. of the BLV particles was about 100 nm. However, in several pellets made from medium of monolayer producing BLV many particles with a diam. of about 80 nm and with surface projections were also found. These smaller particles were not observed in thin sections from the cell cultures and we therefore investigated whether the calf serum used for the preparation of the culture medium contained these particles.
In this report we describe virus-like particles found in nine different bovine sera from commercial suppliers used for tissue culture.”
“All samples of bovine serum examined contained virus-like particles of a similar morphology even after inactivation of the sera for 30 min at 56 °C. These particles were numerous in all preparations. After negative staining the particles were easily recognized by the surface projections of about 11 to 12 nm. The particles were spherical with an overall diam. of 70 to 95 nm (Fig. l). In thin sections of the pellets, the same particles were found surrounded by granular material (Fig. 2). The surface projections of about 11 to 12 nm were also seen in the majority of these preparations.”
“After negative staining BSV has a morphology similar to the presently described particles and it also has surface projections of about the same length (Malmquist et al. I974). The diam. of BVDV is similar to our virus particles, but it is not known yet whether BVDV has surface projections (Dutta, Shope & Pomeroy, I965) or not (Ritchie & Fernelius, I969).
Because it was difficult to determine by morphological studies alone the type of virus these particles represent-if these particles are indeed a virus-a double diffusion test in 0.7 % agarose gel was carried out with samples of each bovine serum against undiluted anti-BVDV, PI-3, IBRV, BSV and BLV sera. Only the sample from Bio-cult Laboratories produced a precipitation line against anti-BVDV serum, but it is possible that the negative results are due to the destruction of the virus antigens during the preparation of the sera for the tissue culture.
When morphological studies are made on virus particles isolated from tissue culture medium, it is important to know whether virus-like particles are present in the bovine serum used for the preparation of the tissue culture medium. This is especially important when the morphological studies are based on negative staining, the morphology of the virus is not well known and the production of the virus is low. We have described as BLV in another paper (Calafat et al. I974) particles isolated from tissue culture media of short-term cultures of leukocytes from cows with persistent lymphocytosis. These particles probably were contaminating particles from the foetal bovine serum.”
https://pubmed.ncbi.nlm.nih.gov/185326/
Finding “virus-like” particles in the media used for cell culture studies should raise serious doubts about the validity of these studies, beyond their inherent pseudoscientific nature and logical flaws. These studies suggest that the particles assumed to be “viruses” after cell culture experiments are simply normal cellular components of the media itself. Once the culture is complete and TEM images are taken, these particles are then interpreted as “viruses.” This forms a circular, flawed system that ultimately creates what it is designed to find. These findings emphasize that the system is self-reinforcing, where the conclusion (that the particles are “viruses”) is built into the methodology, rather than genuinely proving the existence of “viruses.”
By the 1980s, it became increasingly apparent that “virus-like” particles were found in normal bodily fluids as well as in tissue and cell culture samples said to be “uninfected.” This continued to raise questions about whether any such particles were truly “pathogenic viruses.” As a result, researchers began to reconsider the nature of these particles. A shift began in the 1970s and 1980s with the term “virus-like particles” being gradually replaced by “vesicles” or “microvesicles” to describe the small particles shed from the surface of cells, typically through membrane budding or blebbing.
Things took a major turn in 1983 when researchers, working with cell cultures of reticulocytes (slightly immature red blood cells), found small 40-150 nm vesicles in their electron microscopy images. These particles were the same size, shape, and density as “viral” particles, even though there was no “viral” material in the cultures. While the “virus-like” particles were originally thought to be cellular debris and/or garbage bags, they were later assigned a theoretical function related to intercellular communication. Even though the vesicles were indistinguishable from “viral” particles, they were given the name “exosomes” in order to separate them from “viral” particles.
As I have previously detailed the early “exosome” studies, I won’t go into extensive detail here. However, it is clear that the same “virus-like” particles with surface knobs, referred to as vesicles in this pivotal 1983 pre-”exosome” paper by Pan et al. presented below, were found in sheep reticulocytes cultured under various conditions. After 36 hours of incubation, these “virus-like” vesicles—eerily reminiscent of “coronaviruses”—were “separated” from the culture medium.
Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor
“Using sheep reticulocytes, surface-labeled with ‘251, we observed that the amount of ‘251-labeled plasma membrane protein precipitated with the specific anti-transferrin-receptor antibody decreases with time in culture in parallel with the loss of reticulum (Pan, Blostein, and Johnstone, 1983). These data suggested that the receptor might be removed from the plasma membrane. To test this possibility the cell surface was labeled with ‘251 and lactoperoxidase (Reichstein and Blostein, 1975) and the cells were incubated for 36 hr at 37°C. Initially, and after 36 hr, the cells were separated from the incubation medium. Plasma membranes were prepared (Dodge et al., 1963) and solubilized, and the solubilized membranes and cell-free postincubation medium were passed through an anti-transferrin-receptor immunoaffinity column as described (Pan, Blostein, and Johnstone, 1983).”
Electron Microscopic Studies Negative Staining
“The cell-free, postincubation medium derived from incubation of antibody-labeled reticulocytes (‘251-antibody or FITC-antibody) was centrifuged at 100,000 X g for 1 hr. The pellet obtained was stained with 1% ammonium molybdate and examined under a Philips 300 electron microscope. In experiments where the cell-free supernatant was first passed through a protein A affinity column, the acid eluate from the protein A column was immediately neutralized and centrifuged to obtain a pellet for staining.”
https://pubmed.ncbi.nlm.nih.gov/6307529/
This study demonstrated that “virus-like” vesicles were isolated from “uninfected” cell culture supernatants and analyzed for their structure and composition. The methodology—ultracentrifugation, “antibody” staining, and electron microscopy—is identical to techniques used in virology to study “virus-like” particles. Over time, these particles, initially referred to as “virus-like,” were reinterpreted as cellular vesicles and later classified as “exosomes.”
In 1985, a paper by the same research team blurred the line even further between “virus-like” particles and what were later labeled as “exosomes.” This time, upon culturing sheep reticulocytes under various conditions, the researchers observed 100-200 nm vesicles and 50 nm bodies—particles that fall precisely within the reported size range of many “viruses.” The description of the 50 nm bodies forming via budding from intracellular vesicles closely mirrors how virologists describe “viral” assembly and release. Additionally, the reported release mechanism—fusion of MVEs with the plasma membrane, followed by the discharge of small vesicles into the medium—is nearly identical to the observations virologists cite as evidence of “viral” budding and egress. Since these findings are based on static electron microscopy images, the direction of vesicle movement (exocytosis vs. endocytosis) is inferred rather than directly observed, leaving room for misinterpretation. Ultimately, the classification of these structures depends on the interpretation of the researchers rather than direct observation.
Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes
“Using ferritin-labeled protein A and colloidal gold-labeled anti-rabbit IgG, the fate of the sheep transferrin receptor has been followed microscopically during reticulocyte maturation in vitro. After a few minutes of incubation at 37 degrees C, the receptor is found on the cell surface or in simple vesicles of 100-200 nm, in which the receptor appears to line the limiting membrane of the vesicles. With time (60 min or longer), large multivesicular elements (MVEs) appear whose diameter may reach 1-1.5 micron. Inside these large MVEs are round bodies of approximately 50-nm diam that bear the receptor at their external surfaces. The limiting membrane of the large MVEs is relatively free from receptor. When the large MVEs fuse with the plasma membrane, their contents, the 50-nm bodies, are released into the medium. The 50-nm bodies appear to arise by budding from the limiting membrane of the intracellular vesicles. Removal of surface receptor with pronase does not prevent exocytosis of internalized receptor. It is proposed that the exocytosis of the approximately 50-nm bodies represents the mechanism by which the transferrin receptor is shed during reticulocyte maturation.”
“Native, washed sheep reticulocytes were incubated with the specific anti-transferrin receptor antiserum at 0°C for 90 min which resulted in maximum labeling of the cells (5). After washing in PBS, the cells were reincubated at 0°C with ferritin- protein A for 45 min (23). The unbound antibody and protein A were washed away at 0°C, and the cells were transferred to 37°C and incubated for a further 30 min in culture medium (5). The cells were pelleted by centrifugation at 12,000 g for 5 min, washed, fixed overnight at 4°C in 2.5 % glutaraldehyde with 0.1 M sodium phosphate buffer pH 7.4 and 0.1% sodium azide. (b) Colloidal gold label. For labeling with colloidal gold, the cells were incubated with antiserum for 90 min at 0°C, washed 2-3 times, and then reincubated at 0°C for a further 60 min with colloidal gold-labeled goat anti-rabbit IgG. After several washes at 0°C, the cells were introduced into culture medium and the temperature raised gradually to 37°C.”
https://pubmed.ncbi.nlm.nih.gov/2993317/
The fact that “exosomes” and “viruses” are particles identical in shape, size, and density is well-documented in the “scientific” literature. As noted in the article Extracellular Vesicles and Viruses – Two Sides of the Same Coin?, many virologists with experience in extracellular vesicle research (and vice versa) have acknowledged this overlap. The standard definition of an extracellular vesicle encompasses most enveloped “viruses,” leading the author to question how “exosome” researchers can be certain they are isolating and quantifying extracellular vesicles rather than enveloped “viruses” in their samples. Likewise, how can virologists be sure they are not detecting similarly sized “non-viral” vesicles or empty vectors during vaccine production?
The reality is that they cannot. It has been widely acknowledged that no reliable method exists to completely separate these identical particles. Since they cannot be distinguished with certainty, researchers using electron microscopy are observing the same particles in cell cultures—regardless of whether “viral” material has been introduced. Their classification and function are assigned based on disciplinary biases related to the field of study rather than clear physical distinctions.
A closer look at the early days of electron microscopy and the foundational studies of virology reveals a disturbing trend of flawed reasoning. Virologists, influenced by filterability studies and unnatural animal experiments, had already decided what a “virus” should be before ever attempting to visualize one. With the advent of electron microscopy, they began examining unpurified materials from diseased hosts, searching for particles that fit their preconceived notion of a “virus.”
However, at no point were these particles—asserted to be “pathogenic viruses”—ever verified using evidence derived from the scientific method in a way that satisfies Koch’s Postulates. Helmut Ruska and others simply selected shapes from the unpurified mixtures that best fit their imaginary “pathogen.” These subjective morphological interpretations became the foundation for identifying every subsequent “virus” particle. This lack of rigorous validation, combined with reliance on preconceived assumptions, shaped virology into the field it is today.
Notably, the same particles—never scientifically proven to be “viruses”—have been regularly found in both unhealthy and healthy tissue, in cell culture samples, and even in the fetal bovine serum used as maintenance media. They appeared where “viruses” weren’t expected and failed to show biological activity where they supposedly should. When these inconsistencies arose, researchers dismissed the particles as normal cellular constituents or debris—or reclassified them as extracellular vesicles or “exosomes”—whenever it suited the narrative.
This circular reasoning has resulted in a never-ending cycle of identifying non-specific, morphologically identical particles as “viruses” without the necessary scientific evidence in support. As demonstrated here, no new experiments were required to expose this fact—only a critical analysis of virology’s own literature, which reveals the pseudoscientific nature of its methods and conclusions.
examined the impact that artificial lighting has on our health. focused on the various factors that can contribute to disease unrelated to fictional “viruses” and genes. also explored how it is environmental toxins rather than “pathogenic viruses” that impact our health. interviewed Dr. Matt Irwin on on PCR testing, AIDS, “Covid,” exaggerated fear of “infection” & the mind-body connection. had 50 questions for Dr. Robert Yoho, who is looking to dismantle the myths of a healthcare system dedicated to healing in his new book “Butchered by Healthcare.”






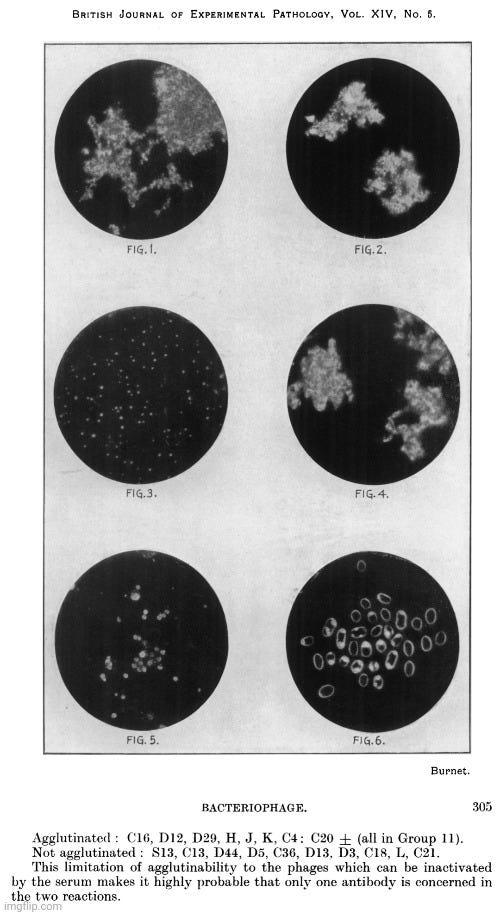

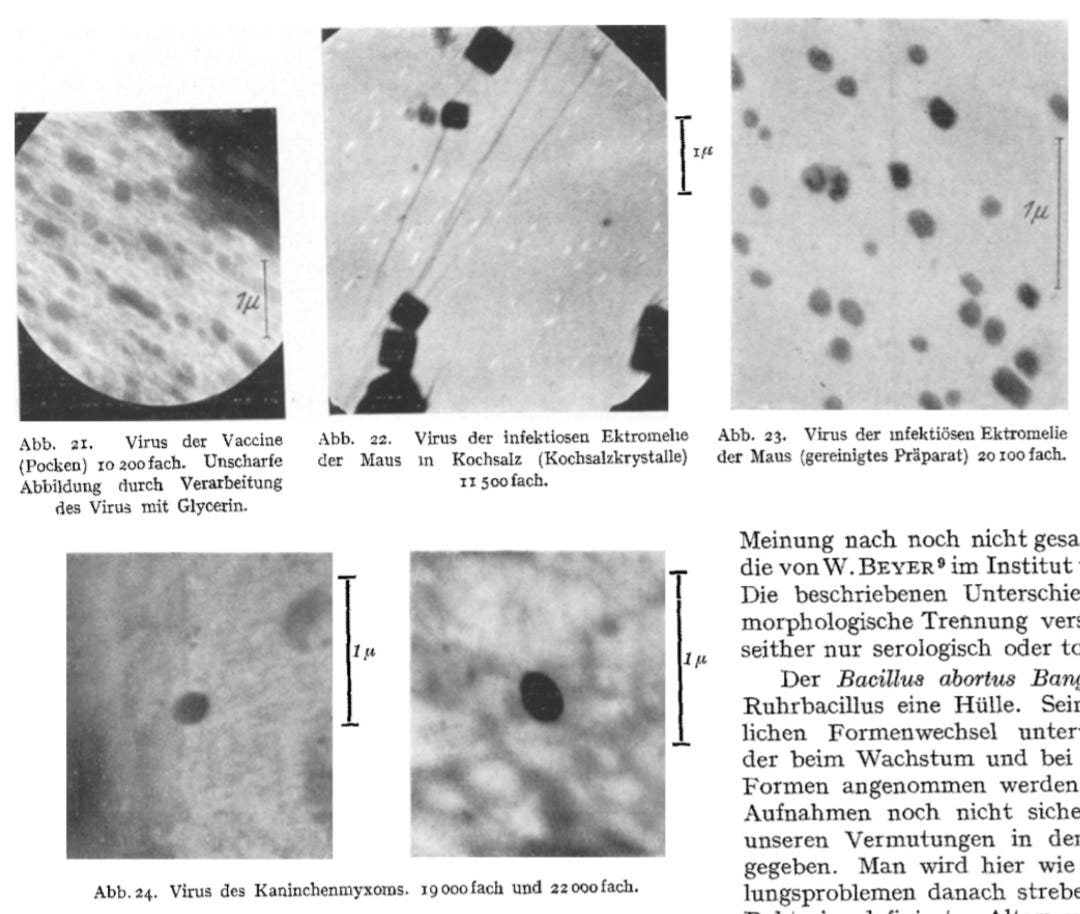

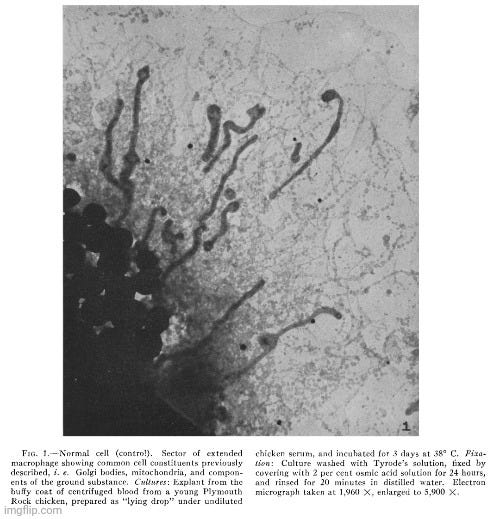

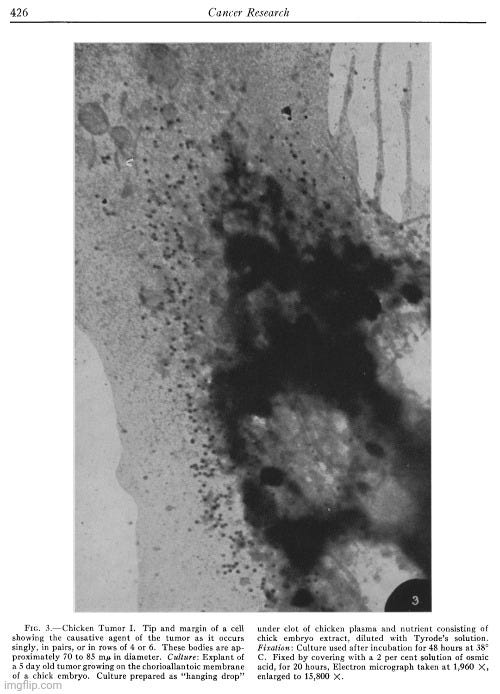
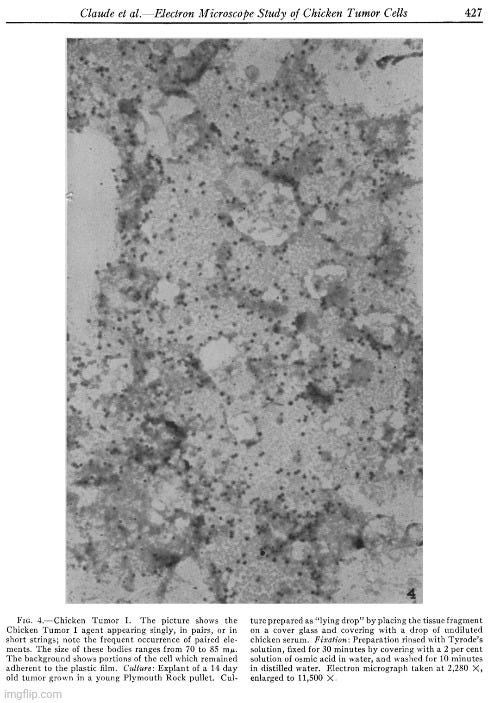

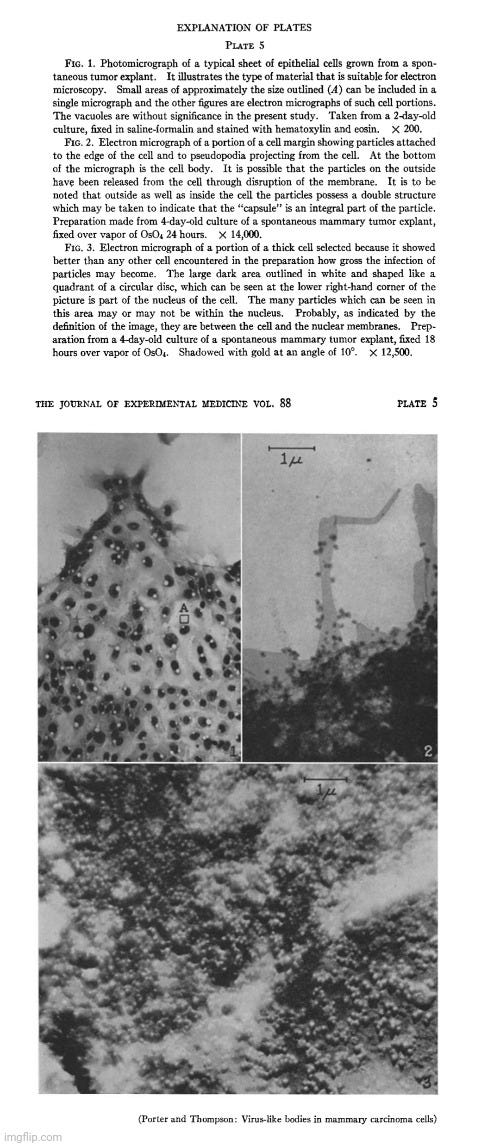
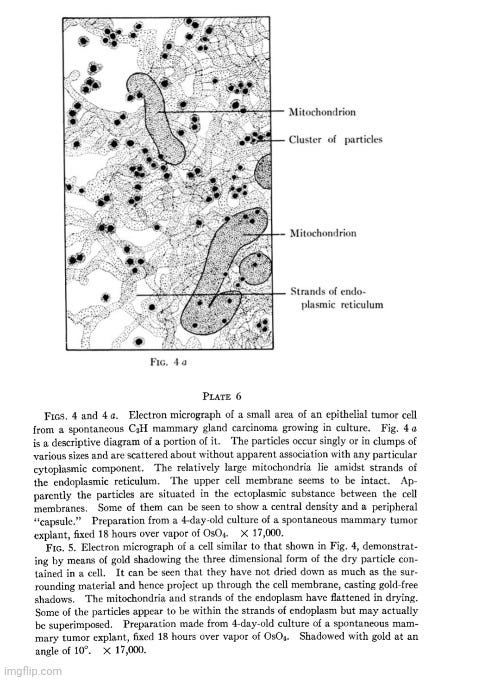
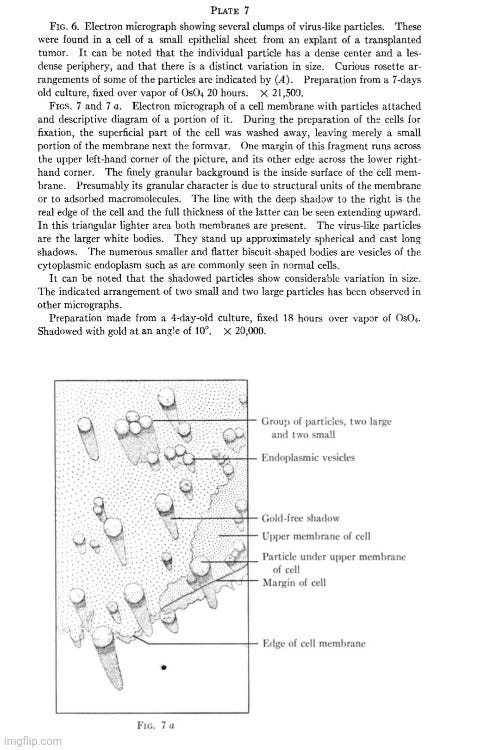
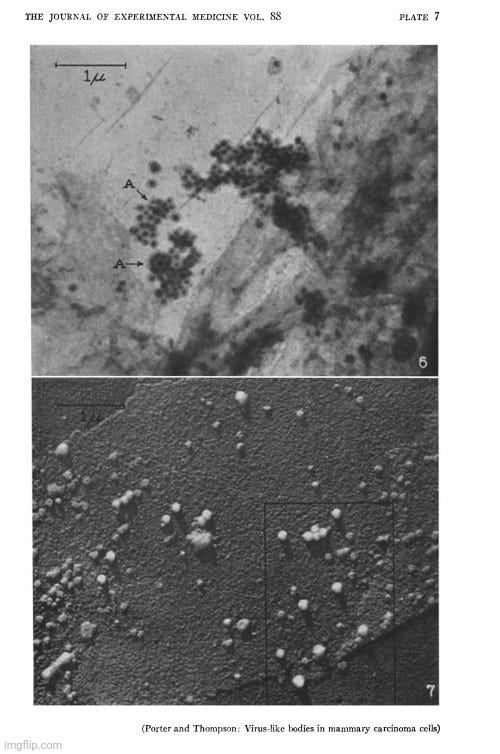

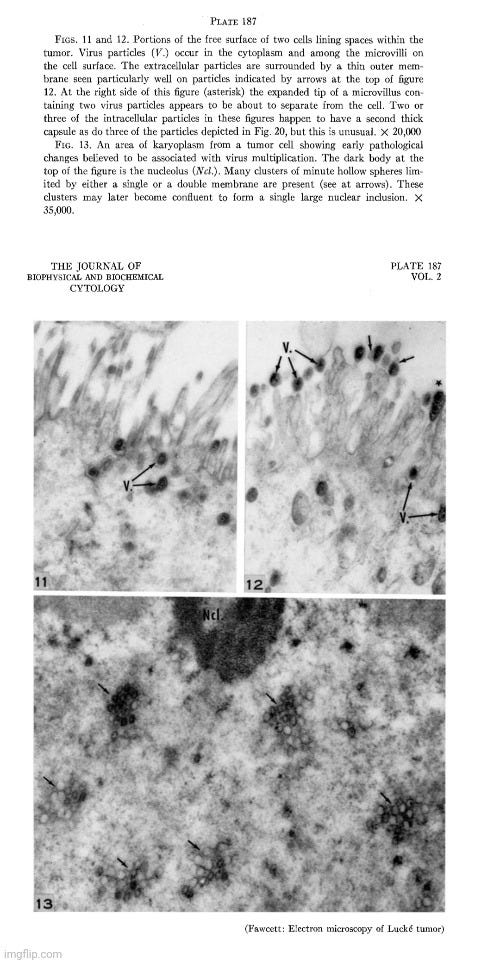

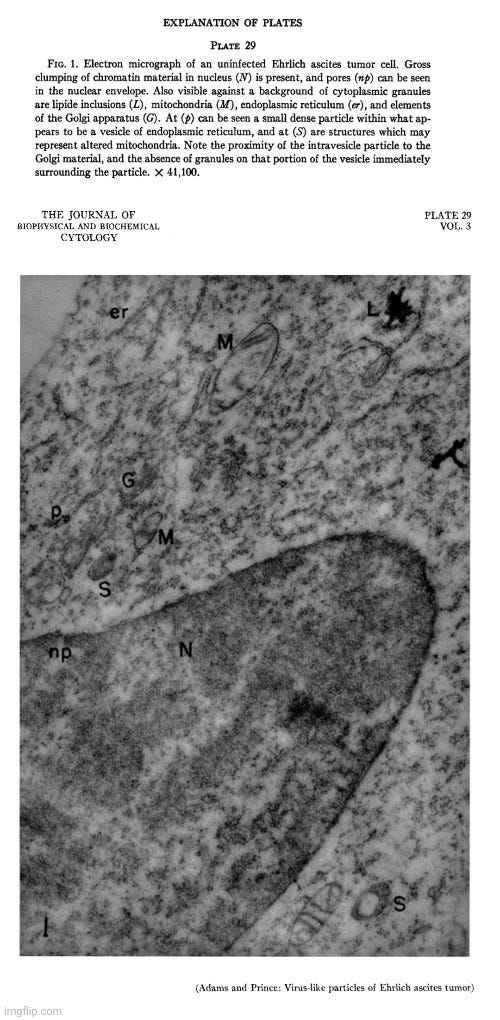
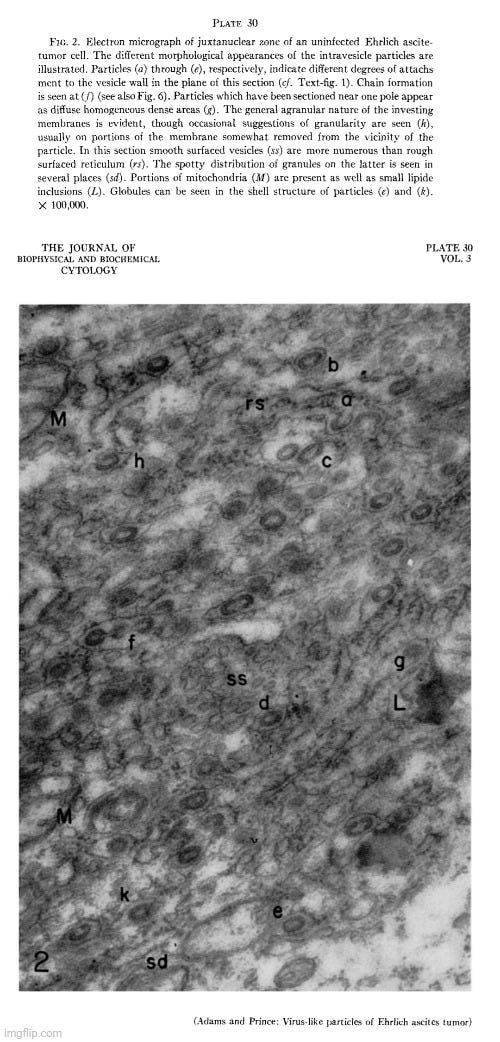
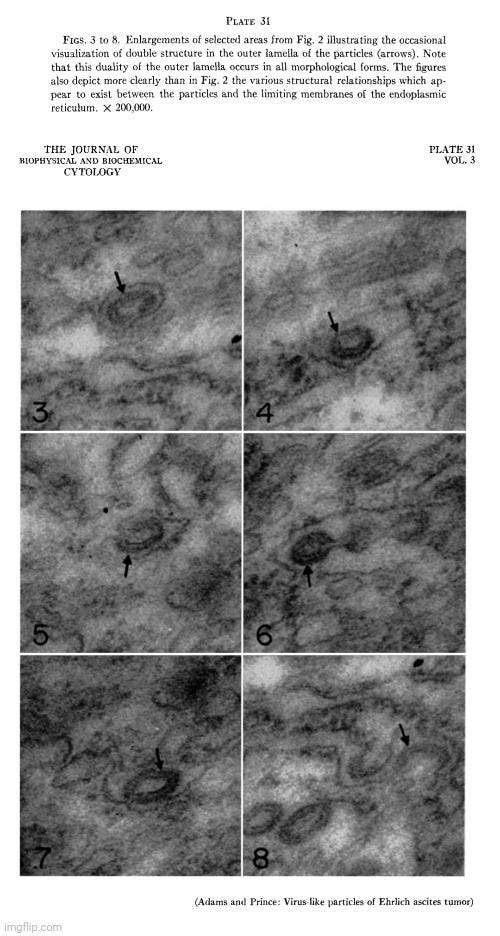

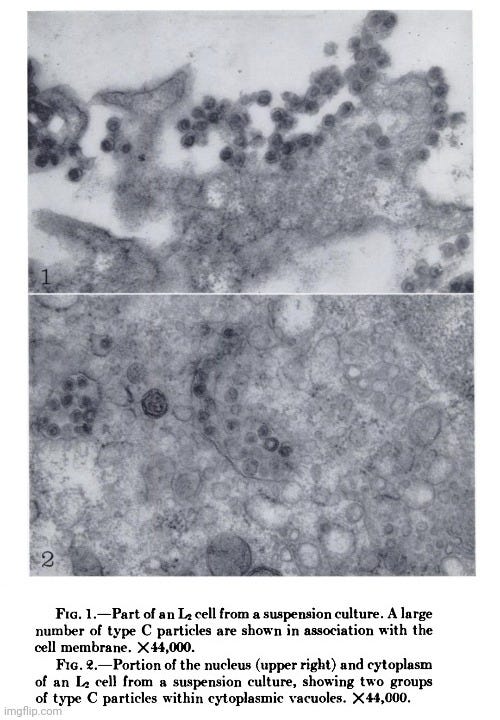


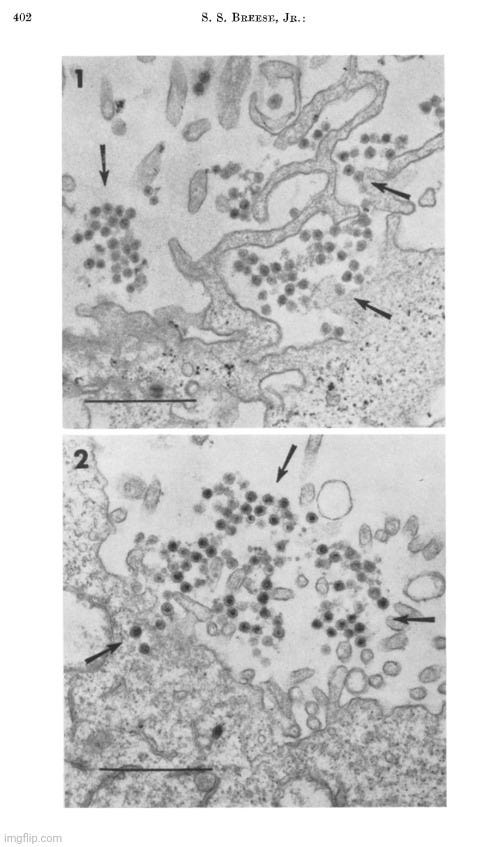





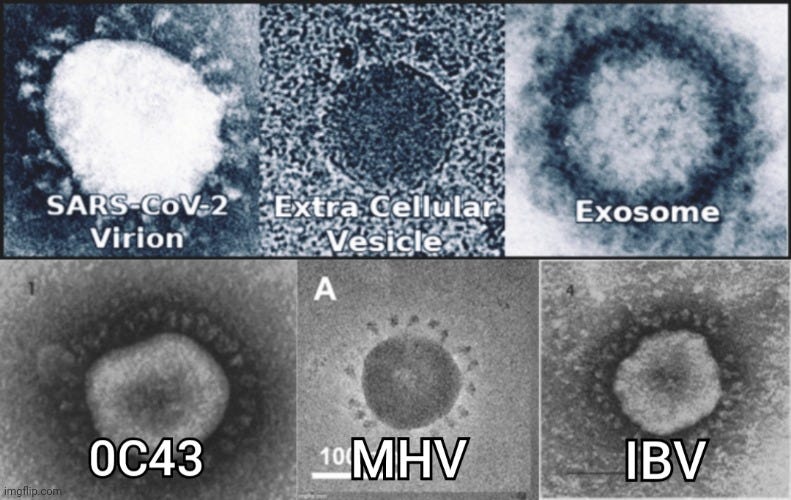






The biggest fallacy probably lies in the fact that for too long people thought that virology was too stupid and too blind to recognize its own mistakes. I think, on the other hand, that everyone involved realized quite quickly that virology leads nowhere. Perhaps there was actually an assumption at the beginning that the visibility of suspected pathogens was still beyond the technical possibilities. But we have been able to overcome this obstacle for several decades now. After that, only deliberate deception can be assumed as a motive. This would mean that the doubters have been too credulous for too long in their assumption that virology would not recognize the errors.
I believe the modern history of virology is a precisely orchestrated lie about control, power and greed. I don't believe for a second that a halfway intelligent person can succumb to self-deception for so long. It takes quasi-religious delusion.
I also don't want to believe that virology is maintained by the stupidest, most naive of us humans. Stupidity is more dangerous than malicious intent. Because no revelations and insights can counteract stupidity.
Bottom line: "Virology" = Quackery. But it sure is lucrative!