The “Virus” Deception: Case Studies in Pseudoscience — Part 1: The “Lipovirus”
How a speculative agent with no direct evidence was framed as a causative "virus."
In the 1950s, virology began to emerge as a distinct discipline, separating itself from bacteriology. For the first time, it was (falsely) recognized as a legitimate scientific field. This shift was largely due to the introduction of the cell culture technique by John Franklin Enders in 1954. This method, still used by virologists today, asserts “viral” presence based on lab-created, artificial effects observed in cultures of human and animal cells.
Around the same time, electron microscopy became more widely available, allowing researchers to peer into unpurified culture soup and label random particles from the destruction of these cells as “viruses.” After decades of vagueness, in 1957, French microbiologist André Lwoff offered a consensus definition for the invisible entities virologists claimed to be studying. Journals were launched to publish the work of these newly anointed experts. It was a decade of enormous “progress” for the pseudoscientific field.
Emboldened by these tools and techniques, “virus” hunters began “discovering” new “viruses” with remarkable ease—despite never having the actual agents in hand to study. But in their rush to interpret indirect, nonspecific, and artificial lab effects as proof of “viral” entities, virologists set the field on a path of misinterpretation, speculation, and pseudoscience.
This pattern soon played out across a range of illnesses in need of a theoretical “pathogen.” One prominent example was hepatitis. According to the editorial Candidate Viruses in Hepatitis, researchers had previously relied on serologic “antibody” testing and human volunteer inoculation in an attempt to prove a “viral” cause for the disease, but the invisible culprit remained elusive. Results that appeared promising for one group were often not reproducible by others—a direct violation of a foundational principle of the scientific method. Yet despite lacking a purified “virus” and failing to replicate findings, researchers still labeled these mysterious agents as “candidate viruses,” signaling nothing more than an unproven suspicion of causation.
“Identification as an agent likely related to hepatitis in turn has relied mainly on serologic (neutralization) tests and inoculation of human volunteers followed by reproduction of clinical features of the disease. A problem precluding maximum accomplishment has been the inability of different research laboratories to reproduce the same results. In addition, essential as repeated isolations from the original material by any given laboratory might seem, this has not always been the case.”
The first “candidate virus” for hepatitis was “discovered” by Werber Henle in 1954 using Maitland-type chick embryo tissue cultures. However, his attempts to demonstrate an “infectious virus” failed: the inoculated volunteers showed no serologic response, and no “immunity” could be observed upon re-challenge.
“The first culture-derived observations on infectious hepatitis were reported by Henle, working with Maitland-type chick embryo tissue cultures. Serological findings in volunteer subjects inoculated with the fluid remained negative, and no immunity could be demonstrated in subsequent challenge with the original agent.”
Over the next decade, numerous labs claimed to isolate “candidate viruses” from hepatitis patients using a variety of human and animal tissue cultures. These included:
Rightsel and Boggs: Claimed hepatitis-like illness was caused by injection of “infected” culture fluid.
Davis: “Isolated” the so-called San Carlos agents (“adenoviruses”) from children’s stool, but no direct link to hepatitis was found.
Chang: Reported a “lipovirus” from hepatitis patients’ blood, but could not classify it or establish any association.
O’Malley: “Isolated” the “A-1 virus” from serum; results were inconsistent, and serologic relationships were unclear.
Bolin et al.: Claimed “isolation” of a “virus” from volunteers given a known serum hepatitis sample.
Hillis: Observed cell damage from hepatitis patient serum, but the effect was lost in serial passage.
Hsiung et al.: Found a “myxovirus” (DA “virus”) in a fatal case, but refrained from claiming causation.
Schneider et al.: Detected agents in both chronic and acute cases using canine lung tissue.
McKees: Claimed to “isolate viruses” on monkey kidney tissue from hepatitis patients, reportedly transmissible to suckling mice.
Despite these numerous claims, no consistent or definitive evidence ever established a direct causal link between any of these agents and hepatitis. Researchers experimented with various tissue and cell cultures, hoping to find a formula that would recreate the disease and produce the expected indirect signs of “infection.” When a particular result appeared to match their expectations, they rushed to assume a “viral” cause. They would name a supposed “virus” and then treat it as if it had been proven to cause the artificial, lab-created effects they had generated. In doing so, they engaged in fallacious reasoning—such as affirming the consequent and post hoc fallacies—deceiving themselves into believing that the invisible culprit was in hand.
Fortunately, some researchers were honest enough to acknowledge when the evidence failed to support early assumptions of a “viral” cause. In many cases, the indirect findings not only fell short of being sufficient—they ultimately pointed away from the “viral” hypothesis altogether. One notable example comes from the work of Dr. Robert Shihman Chang, a pioneer in cell culture. He was among the first to establish immortalized lymphoid cell lines using the “Epstein-Barr Virus,” focusing primarily on developing cell lines that could support “viral” growth for in vitro study. During his investigations into hepatitis, which led to the emergence of the so-called “lipovirus” in 1960, he inadvertently demonstrated how reliance on indirect evidence—such as CPE, “antibody” tests, and electron microscopy—paired with fallacious reasoning can lead researchers to mistakenly believe they’ve discovered a new “virus,” only to later realize the trail of pseudoscientific breadcrumbs led nowhere. This is the story of how a virologist was both misled by assumptions and deceived by the very methods believed to prove a “viral” cause.
This article delves into some technical details. To fully grasp its significance—especially for those unfamiliar with the fallacious and pseudoscientific methods commonly used in virology—it's helpful to review a few foundational articles first. These provide essential background and context to better understand the depth of the deception involved.
I recommend starting with the following:
Reading these will offer a clearer lens through which to view the flawed reasoning and assumptions that underpin the virological narrative exposed in this article.
In 1954, Dr. Robert Shihman Chang set out to investigate the cause of hepatitis—a disease whose origins had eluded researchers for decades—after establishing an epithelial-like cell line from a human liver. At the time, virology was a burgeoning field driven by the belief that many unexplained diseases were caused by hypothetical filterable “viruses”—entities too small to be seen, isolated, or directly characterized, yet presumed to exist based on indirect effects like cytopathic changes in cell cultures. Within this framework, virologists interpreted cell damage as evidence of “viral” activity without meeting the necessary criteria for proper isolation or direct identification of the agent involved. Dr. Chang followed this pattern. Assuming hepatitis was caused by a “virus,” and presupposing the existence of such a “virus,” he attempted to cultivate it in liver-derived human cells.
His efforts to propagate this presumed “hepatitis virus” were detailed in a 1961 paper titled Properties of a Transmissible Agent Capable of Inducing Marked DNA Degradation and Thymine Catabolism in a Human Cell. Though he ultimately failed to isolate or identify the “virus” he was searching for, Dr. Chang reported the unexpected appearance of a “transmissible cytopathic agent” of unknown origin—an observation that, despite its ambiguity and the absence of “viral” isolation, was treated as virologically significant and worthy of further pursuit.
Properties of a Transmissible Agent Capable of Inducing Marked DNA Degradation and Thymine Catabolism in a Human Cell
Following the establishment of a strain of epithelial-like cells from a biopsied specimen of human liver in 1954 (1), attempts were made to propagate the infectious hepatitis virus in this cell. A transmissible cytopathic agent appeared during one of those attempts. Although the origin and identity of this agent has not been ascertained, its many unusual features, its history and general properties are described in this report. The unique property of inducing marked DNA degradation and thymine catabolism in the “liver” cells is described in an accompanying report (2).
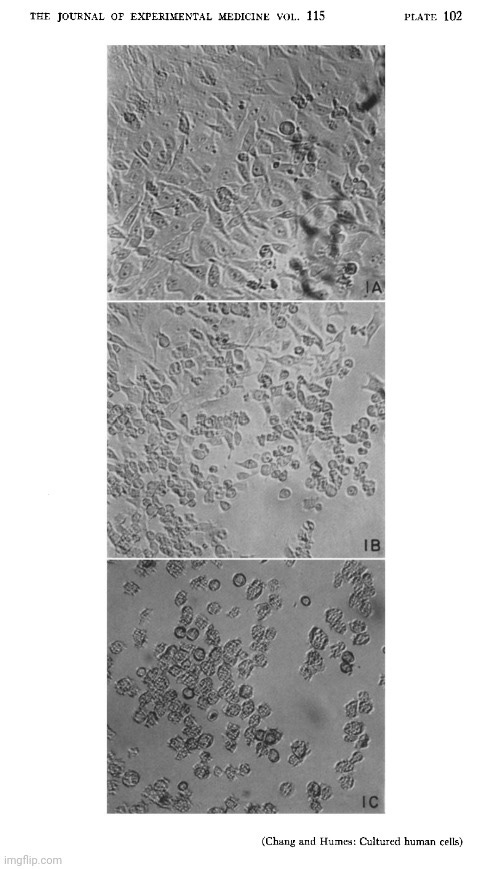
As with all cell culture experiments before and after, Dr. Chang did not purify or isolate any presumed “viral” particles prior to experimentation. He neither identified nor confirmed the presence of a “viral” agent using electron microscopy. Instead, he assumed such an agent existed in the samples obtained from hepatitis patients. The only images presented in the paper were produced via light microscopy of stained cell culture preparations after the fact. These images show morphological changes (Fig. 1) and particulate material (Fig. 2), which Dr. Chang interpreted as being associated with an “infectious agent,” but the images themselves are non-specific. There is no direct visualization of a “virus,” nor any indication of the high-resolution detail that electron microscopy would provide.
The cell cultures were grown in nutrient media containing up to 57% horse serum—a biologically active, non-sterile substance full of undefined proteins, host materials, nucleic acids, “exosomes,” etc. This introduces unknown variables and undermines any attempt to attribute observed cellular effects solely to a presumed “virus.”
To establish the cell line, Dr. Chang used multiple animal and human biological materials, including chick plasma, chick embryo extract, and various human sera. This biological soup invalidates any claim of system purity and introduces many potential sources of foreign genetic material and contaminants—as demonstrated by the discovery of pleuropneumonia-like organisms (PPLO) in the culture in 1956. Although the contamination was “apparently” cleared by prolonged tetracycline treatment and certain culture tests, the sterility and reliability of the model remained questionable.
Moreover, the use of tetracycline to “purge” the culture highlights the dependence on artificial interventions to maintain the system. Antibiotics themselves can induce cellular stress, damage, and behavioral changes—confounding variables that further challenge the interpretation of any causative role attributed to a “virus.”
Materials and methods. Cell cultures. Monolayer roller cultures nourished in a nutrient medium consisting of 57% horse serum, preheated at 56°C for 30 minutes, in Eagle’s basal medium have been used exclusively since 1956 (3). Prior to 1956, a nutrient medium consisting of 20% heated horse serum in Scherer’s MS(4) was used. Chick plasma, chick embryo extract and several human sera had also been used in establishment of this cell line. The “liver” cell culture (1) was found contaminated with a pleuropneumonia-like organism in 1956. Since 1957, following prolonged treatment with tetracycline, the culture has been apparently free of any such contaminants when tested on Edward’s serum-agar (5) , nutrient agar containing 15% horse blood and thioglycollate broth.
Dr. Chang described how, in December 1954, he inoculated four liver cultures with blood drawn from four hepatitis patients during an outbreak in a state institution. Thirty days later, he performed a blind passage, pooling these four cultures into a single liver culture. After 20 additional days, cytopathic effects (CPE) appeared, and these effects could be transmitted to other cultures by adding degenerated cells from the affected one.
History of the agent. In December 1954, 4 “liver” cultures were inoculated with blood freshly drawn from 4 hepatitis patients. The blood specimen was collected on the 1st or 2nd day of jaundice during an outbreak of infectious hepatitis in a state institution for mentally defective children. About 30 days later, a blind passage was made by transferring cells and media from these 4 cultures into one single “liver” culture. In 20 more days, rapidly progressing cytopathic change appeared. This cytopathic change may be readily transmitted to other liver cultures by transferring some degenerated cells. More than 100 serial passages have been made. The data to be described are based on studies made in 1959 and 1960 on this agent which has undergone numerous passages in the “liver” culture since 1954. Unless specified otherwise, the “liver” culture was used exclusively in experiments described here.
The observed degeneration—cytoplasmic granularity, rounding, shrinkage, and distinct nuclear lesions—occurred rapidly after exposure. Some “infected” cultures, however, exhibited inconsistent behaviors: prolonged incubation periods, “chronic” infections with ongoing CPE, or even recovery with new colonies, only to degenerate again with re-exposure. Dr. Chang admitted that the causes of these variable outcomes remained unknown.
Results. Cytopathology. Following the transfer of 0.2 ml of medium and degenerated cells from a completely degenerated culture (previously infected with the agent) to a healthy culture, degeneration usually appeared in 1 or 2 days and progressed rapidly to completion within 2 or 3 days. The degeneration is characterized by cytoplasmic granularity followed by cell rounding and shrinkage. Infected shrunken cells apparently remained intact morphologically for at least 4 weeks when kept at 36°C. Stained preparation of partially degenerated cultures showed characteristic nuclear lesion consisting of basophilic globular bodies frequently situated next to the nuclear membrane (Fig. 1). These basophilic globules are Feulgen positive, and are regularly seen in infected “liver”, infected mouse fibroblasts (strain L) and in those successfully infected primary human amniotic cells.
Due to factors yet to be identified, the infecting process may at times show 3 other features: (1) marked prolongation of the incubation period; (2) establishment of a “chronic” infection, and, (3) reappearance of colonies of healthy cells in apparently completely degenerated cultures. In 2, the culture appeared partially degenerated but continued to shed infectious material for at least 2 months and was capable of converting thymidine-2-C14 to C1402. In 3, the colonies which re-emerged were found destroyed following reintroduction of fresh agent.
Dr. Chang noted that they were able to induce CPE in various cell lines, including conjunctival, HeLa, KB, 1L and appendix derived from human tissues, and mouse fibroblast, strain L, and sarcoma I80 II of murine origin. However, trials using postnatal human amniotic cells and renal cells or simian renal cells failed to produce distinct CPE.
Susceptibility of different types of cells in vitro. Using an inoculum capable of causing complete degeneration of the “liver” cells in 3 or 4 days, cytopathic changes leading to complete destruction were produced in the following cell lines: conjunctival, HeLa, KB, 1L and appendix derived from human tissues, and mouse fibroblast, strain L, and sarcoma I80 II of murine origin. Interestingly, when infected with a similar dose, primary explants of postnatal human amniotic cells (more than 10 trials) and renal cells (2 trials) or simian renal cells (3 trials) failed to show distinct cytopathic changes. With a larger infective dose, the primary amnion culture may also show characteristic degenerative changes.
Dr. Chang was able to reproduce CPE in multiple human and murine cell lines, but some—like human amniotic and renal cells—showed resistance unless exposed to larger doses. These inconsistencies raise questions about the presumed specificity and “infectiousness” of the invisible agent.
Notably, while Dr. Chang's presumed agent produced degeneration in vitro, it failed to induce illness in live animals (mice, rabbits, chick embryos). It also couldn’t be cultured on standard bacterial media, wasn’t affected by antibiotics, and repeated attempts to isolate it from frozen samples were unsuccessful.
Other properties. This agent does not produce any visible illness in baby or adult white Swiss mice by intracerebral or intraperitoneal routes. Chick embryos survive infection by the yolk sac route, and rabbits, by intravenous or intraperitoneal routes. When infectious material was plated on blood agar or Edward’s serum-agar medium and incubated in an atmosphere of 5% COS in air at 36”C, no visible growth could be detected after 7 days. Similarly, no detectable growth appears in thioglycollate broth. Addition of tetracycline at 10 units per ml medium does not affect the progression of the cytopathic changes.
Failure to isolate similar agent. Several attempts to reisolate a similar agent from the 4 original specimens kept frozen at below -60°C were unsuccessful. Similarly, several other frozen acute hepatitis bloods and pooled stool specimens from 2 epidemics of infectious hepatitis failed to yield any transmissible cytopathic agent. It must be emphasized, however, that these attempts were made with frozen material and that freeze-thawing readily inactivates this agent. Isolation study using fresh acute hepatitis bloods is currently being made.
Dr. Chang's explanation for the failure to “reisolate” his “transmissible cytopathic agent” is a textbook case of begging the question. Rather than acknowledging the failure to demonstrate the existence of a transmissible agent, the failure is excused by assuming its existence—and then attributing its absence to fragility. But this assumes what has never been empirically demonstrated: that such an agent exists, that it is sensitive to freezing, and that it was present in the original samples. Since no purified entity was ever isolated or confirmed, these explanations are speculative, not evidentiary.
This kind of reasoning exemplifies a broader issue in virology: assuming a “virus” exists because cytopathic effects are observed, then using that assumption to explain away negative results or gaps in proof. Analogies to known “viruses” are often invoked, despite the fact that those, too, rest on the same unverified assumptions. The circle remains unbroken—evidence is inferred from effects, the effects are attributed to an unproven cause, and contradictory findings are treated as puzzles to be solved within an unquestioned framework rather than as falsification of the hypothesis.
Dr. Chang acknowledged that the identity and origin of the agent remained unknown. Serological tests were inconclusive. Extraction attempts were ongoing. And yet, he still speculated that it might be related to other “viruses”—even as he admitted that its features were unusual for any “known human-infecting virus.”
Discussion. Data obtained to date fail to establish the origin and identity of this agent. Serological findings obtained thus far neither establish nor exclude its immunological relationship with the infectious hepatitis virus. Various methods for release of this agent from the specifically involved shrunken cells are currently being investigated so that neutralization tests may be more adequately performed. Irrespective of its origin and identity, many of its characteristics are unusual for a virus infectious for human cells. These unusual features are: appearance of large basophilic Feulgen positive globular bodies in the nucleus; greater susceptibility of established cell lines as compared to the primary amnion or kidney; complete association of infectivity with the specifically involved shrunken cells; remarkable stability of infectivity of the shrunken cells and apparent rapid loss following procedures which interrupt its structural integrity, such as freeze-thawing and homogenizing, and induction of host DNA degradation and thymine catabolism, described in the accompanying report (2). Certain resemblance to the varicella-zoster group of viruses may be mentioned. The association of the infectivity with the degenerating cell and loss of infectivity following freeze-thawing and homogenizing have been reported for tissue culture propagated varicella-zoster viruses (6). Absence of eosinophilic intranuclear inclusions, failure to demonstrate viral proteins specific for varicella by the complement-fixation tests and the reaction of the cultured cells, to name only a few factors clearly establish the non-identity of the varicella-zoster group of viruses and this agent.
In any other scientific field, the inability to isolate, identify, or trace a proposed agent would be fatal to the hypothesis. But in virology, such uncertainty is tolerated—so long as the word “virus” remains in play.
Ultimately, in his initial investigation, Dr. Chang described a “transmissible cytopathic agent,” even though the presumed agent remained unidentified and unisolated—undermining any firm conclusions about its nature or cause. The observed cellular degeneration was assumed to result from an “infectious” agent, yet no direct evidence confirmed this—only correlation within an artificial laboratory setting. The concept of a “virus” was inferred from the transmissibility of CPE and its disruption by physical stressors, not from direct observation or isolation. Thus, the study assumed “viral” causation based on indirect effects without proving the existence or identity of any “virus”—highlighting foundational weaknesses in the logic and methodology of virology.
Summary. The appearance of a transmissible cytopathic agent during the first blind passage of pooled human “liver” cuitures previously infected with 4 acute infectious hepatitis bloods is described. The infectivity of this agent in the “liver” culture was cell-associated. The infective degenerated “liver” cells retained their infectivity for at least 16 weeks at 4OC and 8 weeks at 36°C‘. Heating for 1 hour at 56°C failed to destroy completely the infectivity of infected mouse fibroblasts. One cycle of freeze-thawing or homogenizing, however, abolished the infectivity with occasional exceptions. Characteristic cytopathology consisted of cytoplasmic granularity, cell rounding followed by diminution of cell volume leading to morphologic picture of shrunken cells. During an intermediate stage of degeneration, characteristic intranuclear basophilic globules were seen regularly: the globules were Feulgen positive. The established cell line was more susceptible to the destructive action of this agent than primary cells. Preliminary immunological study failed to establish the identity of this agent.
As Dr. Chang began his investigations with the preconceived belief that individuals suffering from hepatitis were afflicted with a “virus,” he presumed this unseen entity could be grown in liver cell cultures. Upon observing cytopathic effects in one culture—and being able to transmit these effects to others—Dr. Chang convinced himself that a “virus” was present, despite failing to isolate the invisible agent or identify it through indirect serological testing. While he stopped short of definitively claiming he had discovered a “virus,” his early observations clearly leaned toward confirming his initial assumption.
In a second paper from 1961, Appearance of Marked DNA-Degrading and Thymine Catabolic Activities in a Human Cell Infected with a Transmissible Agent, Dr. Chang continued his investigations by examining the properties of his “unidentified transmissible cytopathic agent.” He described the appearance of DNA-degrading and thymine catabolic activities in “infected” liver cultures. After running tests, his team noted extensive degradation of host DNA into acid-soluble nucleotides—effects that resembled those reported in bacterial “virus” (phage) systems. However, the induction of reductive catabolism of thymine and uracil by a transmissible agent had not been described before. These changes had not been observed in uninoculated cultures, in cultures killed by freezing or nutrient depletion, or in cultures “infected” with other “viruses.”
Appearance of Marked DNA-Degrading and Thymine Catabolic Activities in a Human Cell Infected with a Transmissible Agent
To our knowledge, such extensive degradation of host DNA into acid soluble nucleotides following infection by any transmissible agents has been reported for a few bacterial viruses( 10). Induction of reductive catabolism of thymine and uracil in a host cell by a transmissible agent has hitherto not been described. The exact mechanism of these induced changes and the nature of this transmissible agent are currently being investigated.
Summary. Following infection by a transmissible cytopathic agent and with the appearance of degeneration, the following were regularly observed for thymidine-2-C14 labelled human “liver” cultures: 1. progressive increase in quantity of Gg4O2 liberated, 2. progressive increase in radioactivity of the acid soluble fractions, and 3. progressive decrease in radioactivity of the acid insoluble fractions. Similar changes had not been demonstrated in uninfected culture, culture killed by freezing or by nutrient depleting, and cultures infected with vaccinia, adeno 3, herpes simplex, polio 1, or Coxsackie B1 viruses. Significant conversion of acid insoluble to acid soluble radioactive compounds was repeatedly demonstrated in uridine-2-C14 labelled “liver” cultures under all the described experimental conditions leading to cell degeneration. Formation of C1402 from uracil-2-C14 or uridine-2-C14 was, however, restricted to cultures treated with this DNA-degrading agent.
The above passage perfectly illustrates how extreme biochemical alterations in cell cultures were attributed to an unseen, undefined “agent” based on correlation, not identification. The effects did not match those attributed to “known viruses,” which should have raised red flags about assuming “viral” causation. Instead, Dr. Chang appeared committed to developing the “infectious virus” hypothesis, even while acknowledging the novelty and unknown nature of the observed phenomena. This reveals a fundamental logical flaw: he presumed the very conclusion he should have been testing, highlighting a case of circular reasoning rather than scientific rigor.
Some might argue that the unusual effects could be caused by an unknown “virus,” but this is an ad hoc assumption—not an evidence-based conclusion. Without isolating and characterizing such an agent, invoking a novel “virus” merely shifts the burden of proof and assumes the very thing in question. Rather than rescuing the hypothesis, this deepens the logical vulnerability in Dr. Chang’s reasoning.
In January 1962, Dr. Chang published a third paper, titled The biological, immunological, and physicochemical characterization of a transmissible agent capable of inducing DNA and thymine degradation in cultured human cells, in which he further detailed the properties of the “transmissible cytopathic agent.” He admitted that the characterization of this agent was greatly hampered by its “existence” in a cell-associated form. In other words, the research team had been unable to purify and isolate the presumed “virus” away from host cell constituents. Nevertheless, he claimed that recent success in obtaining it in a “cell-free” form greatly facilitated its characterization.
However, this claim was misleading. While Dr. Chang described obtaining the agent in a “cell-free” state, the actual procedure—by his own description—showed that the material was never truly cell-free in any rigorous scientific sense. The starting material came from cultures that had undergone complete degeneration (i.e., extensive cytopathic effect), meaning the medium was already contaminated with cellular debris, breakdown products, and potentially toxic byproducts from dying cells. The supposed “cell-free” nature of the preparation refers only to the removal of some larger particles via centrifugation—not to a genuine elimination of all cellular material.
Specifically, the team centrifuged the medium at 2,000 RPM, removed the supernatant, and then further centrifuged it at 14,000 RPM to concentrate what they claimed was the “infectious agent.” However, this sediment could still contain:
Fragments of dead cells
Membrane vesicles
“Exosomes”
Protein aggregates
Other stress-induced byproducts
As had been the case in the previous two papers, at no point did the researchers purify or independently verify the existence of a discrete, “infectious agent” prior to the cell culture experimentation or afterwards apart from this complex and toxic mixture. Any “infectivity” observed could just as easily have been due to residual toxins or stress-induced cellular breakdown products—not a “virus.”
The biological, immunological, and physicochemical characterization of a transmissible agent capable of inducing DNA and thymine degradation in cultured human cells
Propagation of this Transmissible Agent.—The history of this agent has already been described (1). Infected "liver" cultures which showed complete cell destruction were found to retain infectivity for at least 10 months when stored at 0-4°C; these cultures served as convenient sources of this agent. To prepare the cell-free form, "liver" cultures nourished in the simplified Holmes' medium were used. After infected cultures showed complete degeneration, they were kept at 36°C for 3 additional days. The media were pooled and centrifuged at 2000 RPM for 15 minutes in a horizontal International centrifuge. The upper 3/4 of this supernatant fluid was collected. Since this fluid was low in infectivity (containing about i0 infectious doses per ml) and contained interferon-like substance as well as a lipogenic toxin (3), it was further centrifuged at 14,000 RPM for 1 hour in a Servall SS-1 angle head centrifuge. The sediment, which contained the infectivity, was resuspended in 1/10 its original volume of simplified Holmes' medium. This concentrated preparation was kept at 0-4°C and may be used for several months. The supernatant fluid which was not infectious, contained the lipogenic toxin to be elaborated upon in the accompanying report (3). In 3 recent experiments, the cell-free form was also obtained from infected "liver," primary amnion, and chick embryonic tissue cultures which were nourished in Eagle's basal medium containing 5 per cent horse serum. The results reported in this manuscript are based on study with this agent, which has undergone numerous passages in the "liver" culture since its original isolation in 1954.
Calling this a “cell-free” preparation of an “infectious agent” is scientifically unjustified and entirely deceptive. At best, the team produced a toxic slurry from dead cell cultures and wrongly attributed its biological effects to a hypothetical “virus.” This is a textbook case of affirming the consequent and false cause fallacies dressed up as virology.
In the 25-page report, Dr. Chang repeatedly cited cytopathic effects—such as the formation of “intranuclear globules” and “nuclear collapse”—as evidence of an “infectious agent.” Yet, as with every virology paper, no independent variable (i.e., a purified, isolated entity) was ever introduced. The assumed cause was never demonstrated to exist apart from the effects it was supposed to cause—an unmistakable case of begging the question.
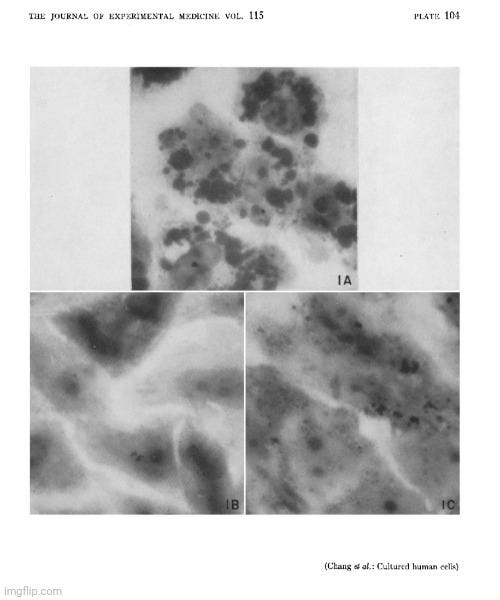
Once again, the published images were not electron microscopy images confirming the purification and isolation of the presumed “infectious agent,” but rather light microscope images of both unstained and stained cell culture material. Dr. Chang explicitly stated that an electron microscopy study of the cytopathology was still in progress—meaning the only technology capable of potentially visualizing the presumed “virus” had not yet been employed. Instead, they simply examined the cell culture supernatant under light microscopes—tools incapable of visualizing particles as small as the hypothetical “virus”—and documented non-specific cytopathic effects as proof.

Despite the claim of “infectivity,” the team found no neutralizing “antibodies” in over 379 human and animal sera. Instead, nearly all tested sera contained inhibitors of “infectivity” that were heat-labile and lipoprotein-like. This would suggest that the so-called “virus” was either always present (or presumed to be), or only appeared “infectious” when normal serum components were diluted. That’s not a “virus”—it’s an experimental artifact.
Dr. Chang also admitted that they were unable to “reisolate” the presumed “virus”—even though no “virus” had ever been isolated from the beginning. This is a direct contradiction of Koch’s Postulates and a fatal violation of the principle of scientific reproducibility. If the so-called “lipovirus” cannot be consistently isolated, its existence is unverified and its identity entirely speculative. Nonetheless, Dr. Chang concluded by hypothesizing that the agent was “DNA wrapped in a lipid coat” and gave it the tentative label “lipovirus”—a claim based entirely on inference rather than direct evidence.
SUMMARY
Experiments designed to characterize an unidentified transmissible agent brought forth the following findings:
The cytopathology consisted of the formation of intranuclear globules, collapse of the involved nuclei, and the extrusion of nuclear materials.
The relatively dormant primary human amnion cells were less susceptible than the rapidly growing cell lines. Similarly, the slowly multiplying ribose variants were less susceptible than their corresponding parent cell lines.
Interferon-like activity was released from infected cells.
Infectivity was readily demonstrated following storage at 0-4°C for at least 8 months or at 37°C for at least 2 weeks. Freeze-thawing, however, markedly reduced or completely destroyed its infectivity.
Infectivity was destroyed completely by ether and chloroform; partially by desoxycholate, and not affected by trypsin, papain, RNAse, DNAse, hyaluronidase, lysozyme, lecithinase, or pancreatic lipase.
The rate of inactivation by 0.025 percent formalin was much slower than that of vaccinia and herpes viruses.
Its synthesis was suppressed by 5-fluorodeoxyuridine. This suppression was not reversed by thymidine and/or uracil.
Heat-stable neutralizing antibody could not be demonstrated in 379 human nd animal serums, in human gamma globulins, or in serums from animals "immunized" with this agent.
Heat-labile inhibitors (lipoprotein-like) capable of inhibiting the infectivity of this agent were demonstrated in 154 of the 157 serums tested.
Experimental evidence indicated the non-identity of this ubiquitous inhibitor and the properdin system.
The non-infectious complex between this agent and the ubiquitous serum inhibitor may be dissociated (hence, become infectious) by simple dilution.
Repeated attempts to reisolate a similar agent have not been successful.
We have hypothesized that this agent is a virus consisting of DNA wrapped in a surface coat rich in lipid, and suggest that this virus be referred to tentatively as a lipovirus.
This report was followed up with a second paper from 1962 titled A lipogenic toxin released through the interaction of a new cytopathic agent (lipovirus) and cultured human cells. Dr. Chang took to formally calling his previously described transmissible cytopathic agent the “lipovirus.” Its name was derived from the assumption—based on indirect evidence—that the agent had a lipid-rich surface coating. This paper introduced a supposed diffusible toxin, found in the supernatant of cultured human cells “infected” with the “lipovirus,” that induced fatty degeneration in human and mouse cells.
A lipogenic toxin released through the interaction of a new cytopathic agent (lipovirus) and cultured human cells
The characterization of a transmissible agent capable of inducing DNA and thymine degradation was described in the preceding report (1). Evidence indicates that the surface coating of the agent is rich in lipids, and for this reason it is suggested that this agent be tentatively referred to as a lipovirus. This report describes the demonstration of a toxic factor found in human cell cultures infected with this lipovirus. This toxin, non-transmissible serially, is capable of inducing severe fatty degeneration of cultured human cells. Preliminary in vivo studies are also described.
The so-called “toxin” was claimed to be present even after centrifugation, which was said to have removed the “infectious” particles. Yet, at no point was electron microscopy used to confirm the absence of these presumed particles, nor was any “virus” identified, isolated, or visualized. The images provided in the study were limited to light microscopy, depicting only general cytopathic effects—such as “sudanophilia” and “cell clumping”—which were merely interpreted as the effects of the hypothetical “lipovirus.”
No direct evidence of a distinct “viral” entity was ever presented. The presence of a “lipovirus” remained entirely assumed.
The cultivation of primary human amnlon or "liver" cells and the production of the transmissible agent were similar to that described in the preceding report (1). The lipogenic toxin was found in the superuatant fluid after the removal of infectious particles by centrifugation at 14,000 RPM for 1 hour in a Servall SS-1 angle head centrifuge; this has been described in the section on the production of the cell-free form of this transmissible agent in the preceding report (1). Unless specified otherwise, toxin prepared from liver cells nourished in Holmes' synthetic medium was used.
Dr. Chang asserted that the toxin was unique to “lipovirus” cultures, as similar toxic effects were not observed in cultures exposed to other “viruses” like vaccinia, herpes simplex, polio, or influenza. He also emphasized that the toxin was dissociable from “infectivity,” distinguishing it from previously described “viral” toxins that are tightly associated with “infectious” particles. Nevertheless, this distinction relied on the unproven assumption that an “infectious particle” was ever present.
Speculative claims about in vivo implications were raised, including the possibility of persistent toxin release leading to fatty degeneration at distant sites. However, the necessary supporting evidence—such as proof of “lipovirus” replication in animals, re-isolation from tissues, or consistent “pathogenic” effects—was lacking. Even the observed liver changes in mice were minimal and based on small, inconclusive samples. The conclusion rested heavily on inference rather than direct demonstration of causal mechanisms.
DISCUSSION
The release of a toxic product capable of inducing fatty degeneration in cultured human and mouse cells from lipovirus-infected cultures appears well established. That this is another unique property of this lipovirus is indicated by our failure to effect the release of a similar toxin from cultures infected by the vaccinia, herpes simplex, adeno 3, polyoma, polio 1 and 2, Coxsackie B1 parainfluenza 1, influenza A, and Rous sarcoma virus. The fact that it is dissociable from infectivity distinguishes it from other viral toxins which are intimately associated with infectious particles.
This demonstration of a diffusible toxin which is capable of producing extensive fatty degeneration in cultured human cells stresses the importance of examining diffusible products of virus-infected cells for biological activities. With the exception of interferon (7) or interferon-like substance (8), published reports on biologically active diffusible products of virus-infected cells are conspicuously meager. There are several reports describing cell-detaching factors from cells infected by the adeno- or polioviruses (9); these factors are neutralized by specific antiserum and are, therefore, presumably specific viral components.
The potential importance of this lipogenic toxin can be comprehended more readily if one takes into consideration other unusual properties of the lipovirus (1). The lipovirus is presumably non-immunogenic, survives in special cells (e.g., sarcoma 180 cells) in vivo for at least 2 months, and presumably does not circulate, owing to the presence of the ubiquitous lipoprotein-like serum inhibitor. It is tempting to speculate whether, in the appropriate in vivo situation, infection with or survival of the lipovirus might be accompanied by slow, continued release of the toxin into the circulatory system with resultant fatty changes at susceptible sites. To substantiate this hypothesis, one would have to establish some replication of the lipovirus in experimental animals, demonstrate its lipogenic activity in vivo, and achieve repeated isolations from clinical materials. Although our preliminary experimental results indicate a slight to moderate increase in sudanophilia of the livers of white Swiss mice receiving this toxin under the described experimental conditions, further in vivo studies are required because of the small number of animals used. Our failure to re-isolate the lipovirus was described in the preceding report (1); the clinical materials used, however, were exclusively the acute phase blood collected from infectious hepatitis patients. Isolation attempts, using other clinical materials, are currently being made. Although chick embryos and white Swiss mice did not develop overt disease following the introduction of the lipovirus (1), it is not known whether limited multiplication of this virus occurs in the apparently healthy animals. Experiments designed to elucidate this point are now in progress.
Despite acknowledging these major evidentiary gaps, Dr. Chang treated the “lipovirus” hypothesis as a given, interpreting correlation as causation and absence of evidence as a prompt to expand speculation. The failure to isolate the agent from clinical materials was not treated as disconfirming, but instead rationalized as a limitation of sampling. This response reflects an ad hoc adjustment that shields the hypothesis from falsification.
In essence, while the paper introduced a novel name and an intriguing toxic effect, the argument rested entirely on indirect inference, unverified assumptions, and speculative extensions—hallmarks of weak causal reasoning. The “lipovirus” remained a theoretical placeholder without direct evidence of its existence or “pathogenic” mechanism.
In June 1963, Thelma Dunnebacke published a paper titled Electron Microscope Observations of Liver Cells Inoculated with Lipovirus, which finally employed electron microscopy in the investigation of the so-called “lipovirus.” However, her objective was not to directly visualize purified and isolated particles from hepatitis patients prior to any cell culture work. Instead, she aimed to observe morphological changes in liver cell cultures following inoculation with material presumed to contain the “lipovirus.”
Dunnebacke received liver cell monolayers grown in Eagle’s medium with 5% horse serum from Dr. Chang. These cultures were inoculated in Chang’s lab using four different “virus” preparations. One set of inoculated and uninoculated cells was sent to the Virus Laboratory, while the remaining three sets were reserved for electron microscopy.
Her observations revealed a variety of cellular alterations post-inoculation—vacuolated nucleoli, chromatin margination and disappearance, and the emergence of spongiform aggregates composed of ring-shaped, electron-dense elements. These peculiar structures originated in the nucleus and migrated into the cytoplasm. Dunnebacke acknowledged that they resembled neither any known organelle nor any previously described “infectious agent.” While she speculated they might be linked to “infectivity” based on their appearance in both intact cells and filtered, cell-free preparations, she admitted their origin was unclear and emphasized that purification would be necessary for proper characterization.
Although the morphology of these structures did not match any known “virus,” Dunnebacke cautioned that their unprecedented, “nonviral” appearance “should not be taken too seriously.” She justified this by citing their relatively uniform size and a form “not wholly unlike recognized virus particles.” This qualification reflects a subtle but telling attempt to reconcile the unfamiliar with established virological expectations. Despite clear visual and structural discrepancies, the interpretation was guided not by direct evidence of a “virus,” but by a superficial resemblance. The hedging reveals a deeper interpretive tension: the unexplained structures were novel and anomalous, yet were still tentatively assimilated into the framework of “viral” causation.
Electron Microscope Observations of Liver Cells Inoculated with Lipovirus
DISCUSSION
The cellular events which followed infection were varied and apparently unrelated. The nucleoli were vacuolated at 2 days, but were no longer seen at 5 days. The chromatin was clearly marginated by 5 days and had disappeared by 9 days. Dense masses of small particles appeared at the periphery of the nuclei and loose aggregates of larger particles appeared in the center of the nuclei at 5 days, but both were no longer found at 9 days. At 9 days, many of the cells were found to contain spongiform aggregates of electron dense material unlike any previously reported cell organelle or infecting agent. The individual ring-shaped elements of the electron dense material originated in the nucleoplasm, formed into aggregates, and migrated into the cytoplasm. Whether an aggregate resulted from coalescence of preformed elements (01 smaller aggregates) or from a process like growth and division of the elements is not known.
The presence of the spongiform bodies in the whole cells shows that the bodies are associated with the cytopathic changes resulting from inoculation of the cells with lipovirus. The demonstration that the infectious cell-free material contains spongiform bodies similar to those seen in intact cells makes it necessary to consider the possibility that those bodies are associated with infectivity. The presence of the spongiform bodies in the cell-free material prepared from two very different types of cell cultures eliminates the possibility that the bodies may be a by-product of infection in a unique cell type. Further progress of identifying these bodies or their elementary units must await their purification.
The complex appearance of the spongiform mass can be accounted for by the large ratio of the thickness of the sections to the diameters of its individual elements estimated to be about 4: 1. If this ratio were 1: 1 or less, as is commonly observed with the larger viruses, the appearance in sections of the elements within the aggregate would not be unlike sections showing elementary bodies in cells carrying the mammary tumor agent (Bernhard et CL, 1955). The unprecedented nonviral appearance of the spongiform bodies should not be taken too seriously, since their elements are relatively uniform in size and have a morphology not wholly unlike recognized virus particles.
Crucially, none of the images presented in the paper depicted purified and isolated particles obtained directly from the host sample, nor were they images of purified and isolated particles from the cell culture supernatant. The observations were confined to intracellular and extracellular debris following inoculation, with no demonstration that any visualized structures had been separated from all other material or shown to possess autonomous ‘infectious” capacity.
Ultimately, the paper illustrates the speculative and assumption-driven nature of many virological interpretations. In the absence of isolation or functional demonstration, ambiguous cellular debris—however morphologically novel—is often assumed to be evidence of “viral” activity. Dunnebacke’s study inadvertently highlights this gap between observation and conclusion, where visual similarity alone is leveraged as indirect support for “viral” hypotheses.
That same month, in June 1963, Dr. Chang published his fourth paper, which focused on investigating the serologic response to his supposed “virus” titled An Immunologic Study of the “Lipovirus.” In this paper, he admitted that it had been virtually impossible to investigate the possible etiologic role of his “virus” in human diseases due to their inability to reisolate the “virus”—one that was never properly isolated to begin with—and their failure to demonstrate “neutralizing antibodies” in human and animal sera. However, the agglutination by “specific” anti-serum of sheep erythrocytes pretreated with the “lipovirus” antigen had been recently observed, and they had also prepared complement-fixing (CF) antigens to the “virus.” The details were described in the paper.
Dr. Chang reported that sheep red blood cells treated with “lipovirus” became agglutinable by specific antisera, and that rabbits injected with the “lipovirus” produced “antibodies” reactive to these treated cells. This was taken as confirmation of the conclusion that “lipovirus” was a new “transmissible cytopathic agent” not described previously by other investigations. However, rabbits injected with “lipovirus” grown in human cells sometimes developed “antibodies” against chick cell components, and vice versa, indicating potential responses to residual host cell material rather than a distinct “viral” entity. Although no serological relationship was found between the “lipovirus” and a host of “known viruses,” this lack of cross-reactivity did not confirm novelty—it could instead reflect non-specific or artifactual responses. Overall, the findings pointed more toward reactions to altered or contaminating cellular components than to clear evidence of a unique, “infectious virus.”
Interestingly, a critical point was raised prior to the summary: the “cell-free” preparation of the “virus” may have still contained numerous cell fragments that were hard to neutralize, suggesting that what was called a “virus” could have actually been a mix of cellular debris. This undermined the claim of a distinct “infectious agent” and supported the interpretation that observed “antibody” responses were directed at host cell remnants rather than a “virus.”
An Immunologic Study of the “Lipovirus.”
The failure to demonstrate neutralizing antibody confirms a previous report (5). Three possibilities were then offered as explanations: a) the outermost surface of this agent is immunologically nonreactive; b) animals injected with this agent did not respond with the formation of specific antibody; and e) methods used for the demonstration of neutralizing antibody were not sufficiently sensitive. The second possibility may now be excluded since animals do respond to immunization with formation of specific antibody and many human sera do contain llpovirus antibody (6). The possibility that the "cellfree" virus preparation still contained many cell fragments which are difficult to neutralize should also be seriously entertained.
The immunologic data described herein and those to be described in the subsequent report (6) appear to further substantiate the conclusion that lipovirus is a new transmissible cytopathic agent not described previously by other investigators.
SUMMARY
Sheep erythrocytes treated with lipovirus were so altered that they became agglutinated in the presence of specific antiserum. The serum agglutinating factor was stable to heat at 56°C for 30 min and was absorbed by llpovirus-treated red cells. This agglutination was specifically inhibited by pretreatment of antiserum with lipovirus. Rabbits injected with lipovirus responded with the formation of antibody in high titers as demonstrated by this reaction. Serums from 28 rabbits injected with a variety of viral, rickettsial, and bacterial agents and cell components did not agglutinate lipovirus-sensitized erythrocytes. Complement-fixing antigen specific for the lipovirus was prepared by concentrating the specific involved shrunken cells. Antibody responses were demonstrated in all rabbits injected with the lipovirus but not in those injected with uninfected cells. Some animals immunized with lipovirus grown in human cells showed rises in antibody against uninfected chick cell antigen. Similarly, some injected with lipovirus in chick cells showed rises in antibody against human cells.
A variety of antisera or paired acute and convalescent sera were tested for "lipovirus" antibody. There is no evidence that the lipovirus is immunologically related to any of the following viruses: vaccinia, herpes simplex, varicella, cytomegalo-, adeno-, SV40, polyoma, influenza types A and B, para-influenza types 1, 2, 3 and 4, measles, mumps, respiratory syncytial and Newcastle disease viruses.
Dr. Chang followed up his initial report with another paper focused on the serological aspects of his proposed “virus,” titled Patterns of “Lipovirus” Antibody in Human Populations, in August 1963. This study examined sera from various groups, including those diagnosed with “infectious” hepatitis. Chang claimed the findings were “quite conclusive” in showing that “lipovirus antibodies” were prevalent in the general population.
Patterns of “Lipovirus” Antibody in Human Populations
“Evidence presented appears quite conclusive that lipovirus antibodies are prevalent in the general human population. Of the 332 individuals residing in the Boston area, 59% were positive in the CF test and 35 % in the SEA test. The high percentage of positives in the youngest age group suggested the existence of trans-placental transfer of maternal antibody. The drop in the incidence of positives in the 1-year-old group (33%) followed by a rise to a peak of 83% in the 5- to 9.9-year group indicated that the infection was acquired primarily during childhood. The decline in the incidence of positives in the older age group can probably be explained by the rather rapid disappearance of serum antibodies (as measured by these tests). For instance, declines in antibody titers from 80, 160 or 320 to 0, 10 or 20 in an interval of about 2 years were frequently observed (see Table II).
The paper framed these findings as evidence of a “novel infectious agent” linked to “infectious hepatitis,” but the claims rested on weak correlations, speculative interpretations, and serious methodological flaws. Again, no “virus” was ever purified and isolated. The supposed antigenicity was never shown to be independent of serum components or cell culture artifacts. Instead, the argument relied heavily on “antibody” detection via complement fixation (CF) and serum electron agglutination (SEA) tests, which were never validated against any purified or characterized “viral” particle.
A major problem was the inconsistent correlation between “lipovirus antibody” titers and actual hepatitis cases. While some patients showed elevated titers or a fourfold rise in convalescence, most did not. Dr. Chang speculated that blood was collected too late to detect an early “immune” response—an ad hoc explanation that underscored the failure to meet even basic criteria for establishing causality.
Further complicating matters, serum from hepatitis patients in a Nevada outbreak showed no difference in “antibody” levels compared to the general population. Rather than challenge the “lipovirus” hypothesis, Dr. Chang instead invoked the existence of multiple hepatitis agents—again, without providing direct evidence.
The incidence of positive tests as well as of high antibody titers was significantly greater in the infectious hepatitis patients than in the non-hepatitis general population. All patients belonging to the Fitchburg, Ethiopian and Fernald State School epidemics and the five sporadic cases were positive at serum dilution of 1:40 to 1:320. In sharp contrast only 36% of the nonhepatitis group of comparable age were positive at similar serum dilution. Furthermore, 80 % of the paired or serial specimens from the Fitchburg epidemic showed declines in antibody titers by a factor of 4 or more during the convalescent and postconvalescent periods. This suggests that the relatively high antibody titers seen in those Fitchburg hepatitis patients are the results of recent infection by an agent immunologically related to the lipovirus. In one of the sporadic cases, there was a rise in lipovirus antibody from 1:10 for a preillness specimen to 1:80 during convalescence. Among the 23 patients suffering from a variety of acute infectious conditions unrelated to infectious hepatitis, the incidence of positives as well as the antibody titers were similar to the general population. All these findings lead toward the hypothesis that the lipovirus is immunologically related to an agent capable of causing infectious hepatitis in man. The high incidence of positives and the age distribution of positives in the general population are also consistent with our present knowledge of the epidemiology of infectious hepatitis in man. The failure to demonstrate antibody rise in practically all the infectious hepatitis patients under study may be due to difficulty of recognizing the disease in its early phase. Most of the first bloods were collected in the 1st or 2nd week after onset of jaundice; at this time, the patient had probably been infected by the hepatitis agent for at least 30 to 40 days.
The observation that sera from hepatitis patients in the Nevada epidemic did not differ significantly from the "general" population with respect to incidence and titer of lipovirus antibody is of interest. It seems possible that there may be more than one agent capable of causing epidemic hepatitis of man and that this particular hepatitis agent bears no immunological relation to the lipovirus.
The failure to demonstrate lipovirus antibody in the serum hepatitis patients suggests that the lipovirus is unrelated to this strain of serum hepatitis agent which was used in infecting human volunteers.
These patterns were interpreted as evidence of recent “infection” with an agent “immunologically related” to the “lipovirus.” However, this assumed the very conclusion they set out to prove—that the detected “antibodies” were responses to a specific “viral infection.” Crucially, the study never ruled out confounding factors.
Perhaps most damaging to the “lipovirus” hypothesis was the acknowledgment that horse serum used in tissue culture could have introduced foreign antigens that triggered “antibody” formation. The presence of “antibodies” in horses themselves, including strong SEA reactions, suggests that the observed “immune” responses in humans may stem from exposure to serum contaminants rather than a novel “viral pathogen.” This possibility, that Dr. Chang admitted had been “entertained for the past 8 years,” was never ruled out, and it directly challenges the validity of the entire study’s assumptions.
Although the finding of weak and inconsistent SEA reactions in the chick sera may possibly be nonspecific, those encountered in four of the nine horse sera cannot be so disregarded. The titers were high and agglutination was strong. In addition, they were also positive in the CF test, although at very low titers of 1:10. One has to accept, at least tentatively, that lipovirus antibody is also present in many horses. This leads to the possibility that the lipovirus may have originated from a certain batch of horse serum which was used as a component of tissue culture medium. It should be emphasized that this possibility has been entertained for the past 8 years, that many batches of horse serum have been used in medium for Lich-1 and many other cells, and that transmissible cytopathic changes have not been encountered in uninoculated cultures.
Despite this serious confounder, Dr. Chang maintained belief in a “novel human pathogen,” even proposing human challenge trials. The discussion reflected classic circular reasoning: presuming a “virus,” interpreting “immune” responses as evidence, and rationalizing away contradictions. The hypothesis was effectively unfalsifiable—no result could have disproven it, since negative findings were always attributed to bad timing, test sensitivity, or alternative agents.
Obviously, much needs to be done to establish the identity between the lipovirus and the agent of infectious hepatitis of man. For instance, surveys for lipovirus antibody in individuals suffering from infectious hepatitis and in appropriate controls should be continued. Serial serum specimens collected from individuals experimentally infected with the agent of infectious hepatitis should be assayed for lipovirus antibody. Attempts should be made to demonstrate lipovirus antigen in the stool, blood and urine of the infectious hepatitis patients. If sufficient justification should arise, experimental infection of human volunteers may be considered. These are, however, beyond the resource of this Laboratory at this time.
Despite contradictory evidence, speculative leaps, and a complete lack of “viral” isolation, Dr. Chang ultimately concluded that the “lipovirus” was either a common “infectious agent” or antigenically related to one. His belief rested not on demonstration but on interpretation—interpretation that was shaped by assumption rather than independent, replicable proof.
It is concluded that the lipovirus is a common infectious agent of man or is antigenically related to a common human pathogen, and that the lipovirus is immunologically related to an agent capable of causing infectious hepatitis in man.
In March 1964, Dr. Chang provided more indirect evidence for his assumed “virus” when he published the paper Temperature Induced Cellular Resistance to the “Lipovirus.’’ In the opening, he reiterated his hypothesis that the “lipovirus” was a “new transmissible cytopathic agent isolated from (and ostensibly present in) the acute phase blood of a patient with infectious hepatitis.” He noted that “antibody” to the “virus” had been found to be “prevalent in man, particularly in those patients suffering from a disease clinically diagnosed as infectious hepatitis.”
In this paper, Dr. Chang attributed nonspecific, temperature-sensitive effects to his “lipovirus” without demonstrating actual “viral” causation. The reported degeneration in cell cultures only occurred at 32–34°C and was suppressed at normal mammalian body temperatures, which actually undermined the claim that a “pathogenic virus” was responsible. A genuine “pathogen” presumed to cause disease in warm-blooded hosts should remain active—or even thrive—at physiological temperatures, not become inert. The fact that the cytopathic effects disappeared at normal body temperature suggests that the degeneration was more likely due to experimental artifacts, environmental stress, or temperature-sensitive toxicity, rather than the activity of an “infectious agent” capable of causing disease in vivo.
Temperature Induced Cellular Resistance to the “Lipovirus.’’
Discussion. The influence of temperature on virus replication has been quite extensively studied (11-13). The results obtained for the “lipovirus” differed strikingly from those described by other investigators. Complete suppression of viral replication occurred in infected cultures at 38.5°C and such cultures retained their infectious potential for at least 35 days. This is of interest because this critical temperature is only slightly higher than the physiological temperature of man and is not detrimental to the survival or multiplication of human cells in vitro. Thus, a relatively minor alteration in cell environment may bring about a complete reversal of cell susceptibility to this very unusual transmissible agent. The mechanism for this complete reversal of cellular susceptibility is not known. It is not due to the release of interferon-like substances or extracellular inhibitors) or due to an increase in the rate of extracellular viral decay. There is no evidence that cellular metabolism was drastically altered. The state of resistance is reversible and, therefore, not due to the emergence of a resistant cell population.
A passage from the “Discussion” section revealed serious flaws in the logic and interpretation of the experimental findings. Dr. Chang observed that raising the culture temperature to 38.5°C—clearly within the range of a physiological fever—completely halted replication of the so-called “lipovirus.” The cells themselves remained viable, and yet the “infectious potential” somehow persisted for over a month. In his words: “The optimal temperature for development of specific degeneration in ‘lipovirus’-infected cultures was 32–34°C. Partial and complete suppression was noted at 37.5°C and 38.5°C respectively.” This is peculiar from a “viral” perspective, as one would expect a “pathogen” capable of “infecting” warm-blooded organisms to thrive at, or at least tolerate, normal human body temperatures. Yet the alleged agent appeared to lose all replicative ability at levels routinely reached during minor fevers. Chang acknowledged that this dramatic shift in susceptibility could not be explained by typical mechanisms like interferon activity, altered metabolism, or resistant cell populations.
Rather than considering that the observed effects might not be due to a “virus” at all, Dr. Chang doubled down on assuming a “viral” cause despite lacking a clear independent variable or mechanistic explanation. The results—temperature-sensitive degeneration and reversibility—are more consistent with a nonspecific or toxic cellular response than with a “pathogenic, infectious agent.”
Similarly, the reported lethality in chick embryos varied depending on both the injection site and temperature, suggesting that the effects were most likely due to methodological artifacts or toxic reactions rather than a specific “infectious agent.” As with every study in support of the “lipovirus,” there was no purified “virus” shown, and thus, no valid independent variable established prior to the experiments, making the claims scientifically unsupported.
Summary. The optimal temperature for development of specific degeneration in “lipovirus”-infected cultures was 32-34°C. Partial and complete suppression was noted at 37.5°C and 38.5°C respectively. The “lipovirus” was lethal to chick embryos if the virus was inoculated into the amniotic or allantoic sacs or onto the chorioallantoic membrane and the embryos then incubated at 33°C. Inoculation into the yolk sac or elevation of incubation temperature to 38°C nullified the lethal action of the “lipovirus” on chick embryos.
In short, Dr. Chang observed unexplained cell changes and, without properly identifying or isolating a “virus,” continued to infer its existence in order to support his hypothesis—a textbook example of begging the question and pseudoscientific reasoning.
A few months later, in June 1964, Dr. Chang published the paper The Continuous Multiplication of Lipovirus-Infected Human Cells. In the paper, he asserted that cultured Lich-1 cells, once “infected” with the so-called “lipovirus,” could be maintained in continuous cultivation for at least two years. These cells retained the abnormalities reported in earlier experiments—such as nuclear shrinkage, lipid accumulation in the cytoplasm, the presence of spongiform bodies, and degradation of at least 80% of cellular DNA.
These findings, however, were not accompanied by the necessary scientific rigor. The “Discussion” section once again illustrates how speculative assumptions can be mistaken for scientific conclusions when proper controls and causal reasoning are absent. Dr. Chang claimed that “lipovirus-infected” cells underwent distinct morphological and biochemical changes, and that under enriched conditions, they gave rise to a continuously replicating, abnormal cell line termed ILEM cells. Even though cells were examined by light or electron microscopy, the presumed causal agent—the “lipovirus”—was never purified, isolated, or independently verified prior to experimentation or examination. Its existence and effect were again inferred solely from the presence of downstream cellular abnormalities.
In this framework, “infection” was redefined as the persistence of altered cellular traits, and “infectivity” was claimed based on the degeneration of co-cultured normal cells—yet no purified agent was introduced. This represents a clear case of circular reasoning: the “virus” is assumed to exist and cause the changes, and the changes are then used as evidence that the “virus” exists in order to support the hypothesis. Alternative explanations—such as chemical toxicity, cellular stress, or selection for damaged but metabolically active cells—were not explored or ruled out.
Most striking is the interpretation of the reduced DNA content in ILEM cells as a meaningful biological insight, suggesting that large portions of DNA might be unnecessary for replication. But rather than representing a revolutionary discovery, this observation is more plausibly explained by experimental artifact or stress-induced degradation.
The Continuous Multiplication of Lipovirus-Infected Human Cells
Discussion.-When Lich-1 cells are infected with lipovirus, they undergo characteristic changes in morphology, antigenic content, and catabolic activity for thymidine and adenine. In standard culture medium these changes signal the death and disintegration of the cells. If, however, the cultures containing the changed cells are placed in an enriched medium, they continuously grow in numbers, at the same time retaining the characteristics of the lipovirus-infected cells. They form a homogeneous population with respect to their abnormal morphology and in this state they may be serially transferred, apparently indefinitely. Such cells, here called ILEM cells, continue to be infective when tested against normal Lich-1 cells. Their average DNA, RNA, and protein contents are distinctly less than those of the parent Lich-1 cells.
The evidence seems good that, indeed, the individual, infected cells are multiplying in the ILEM cultures: (1) all cells examined, either by light or electron microscopy, show the morphological changes appearing in infected Lich-1 cultures (at 32-34°) 7 days after inoculation; (2) binary fission has been documented by time-lapse cinemicrography; (3) the ILEM cultures are shown to contain the lipovirus by antigenic tests and by infectivity assays; (4) uninfected cells, kept under conditions which support the multiplication of ILEM cells, degenerate completely within 14 days. These facts seem to rule out the alternate possibility that the ILEM cultures increase in cell numbers because of the presence of a few "contaminating" normal cells which continue to multiply and to provide new cells for infection.
The drastically lowered DNA content of the ILEM cells, as contrasted with the parent Lich-1 cells, is a surprising observation, suggesting the possibility that not all the DNA content of the parent cells is necessary for the maintenance of cell integrity and replication.
As with Chang’s previous studies, the paper substitutes assumption and interpretation for direct demonstration. The methodology failed to establish a clear causal chain, instead relying on a self-fulfilling logic in which a “virus” is “found” precisely because its existence was assumed from the start.
Although Dr. Chang spent years attempting to convince both himself and others that he had been working with a “virus” since 1954, cracks in this hypothesis began to emerge more visibly by 1966. In July of that year, Dr. Chang published his final “lipovirus” paper On the Nature of the “Lipovirus,” where he opened with a striking admission: the true nature of the so-called “lipovirus” remained uncharacterized and unknown.
Far from describing a clearly defined “viral” agent, Dr. Chang conceded that the “lipovirus” appeared to be a transferable factor—or set of factors—carried by an ameboid cell and transferred to human cells through an unknown, contact-dependent mechanism. This process diverged sharply from classical virology, which defines “viruses” as cell-free, filterable agents that replicate through “well-established” pathways. Instead of presenting direct evidence of a “virus,” Dr. Chang described a phenomenon involving abnormal cellular changes, antigen accumulation, and cell disintegration, all without isolating or purifying a causative agent.
Notably, he acknowledged that the current criteria for defining a “virus” had not been met, yet he persisted in using the term “lipovirus” in quotation marks to avoid introducing a new name that might create confusion. Ironically, this cautious use of virological language perpetuated the very confusion he sought to avoid. In doing so, Dr. Chang maintained a semantic bridge to virology that lent unwarranted legitimacy to what was, in essence, a “non-viral,” cell-associated phenomenon. By labeling an ill-defined and speculative process with virological terminology, Chang continued to risk misleading interpretations and conflating correlation with causation. What he ultimately presented was not a discovery of a “virus,” but a pseudoscientific hypothesis concerning a poorly understood lab-generated phenomenon—calling for further investigation rather than establishing proof. It’s a textbook example of how ambiguous terminology, when left unchallenged, can shape scientific narratives in the absence of concrete evidence.
On the Nature of the “Lipovirus”
During an attempt to isolate viral agents from the bloods of infectious hepatitis patients, an unusual transmissible agent was encountered (1). Since the activity of this agent was apparently protected by a lipid-rich outer membrane, and since a lipogenic toxin was consistently found in inoculated human cell culture, the name "lipovirus" was proposed tentatively (2, 3). This name was subsequently used with quotations to signify the uncertainty of and the necessity for further investigation on the nature of this agent.
Experimental data which lead to the formulation of the following concept have now been obtained. The "lipovirus" is a transferable factor(s) which is carried by an ameboid cell. The ameboid cell is capable of transferring the factor(s) to human cells by an unknown mechanism which requires cell-to-cell contact. Some human cells which have accepted the factor(s) develop characteristic nuclear changes, accumulate specific antigens, and eventually disintegrate. The exact nature of this transferable factor(s) remains to be investigated. There is no direct evidence that the recipient human cell can transfer the factor(s) to other human cells, or that the factor(s) can alter a human cell into an ameboid cell. Data in support of this concept are presented in this and the accompanying mauscript (4).
To avoid introducing a new name for a transferable factor(s) whose exact nature remains to be elucidated, we would like to suggest that the term "lipovirus" (used with quotations) be temporarily retained for this transferable factor(s), and that the, ameboid cell carrying this factor(s) be temporarily referred to as the ameboid cell-"lipovirus" complex, or, simply, AL complex. We are aware of the fact that the characterization of the "lipovirus" is still incomplete and that the criteria proposed currently for a virus (5) have not yet been fulfilled. However, to introduce a new and temporary name for this factor at this time would certainly cause further confusion in this area of investigation. It should be emphasized that the main objectives of this manuscript are to clarify some of the confusion relating to this complex phenomenon and to interest other investigators in examining this phenomenon critically.
Dr. Chang took to calling his artificial phenomenon the “Ameboid Cell–’Lipovirus’ Complex” (AL complex), and further elaborated on this by detailing a process that stood in stark contrast to the established standards of virological research. He revealed that the phenomenon was first observed in 1954 when freshly obtained hepatitis blood was added to newly established Lich cell cultures. Yet all subsequent efforts to reisolate the agent failed—an admission that undermines the reproducibility essential to science.
The AL complex was propagated solely through serial passages in Lich cultures, using unfiltered fluids from previously inoculated cultures that had shown cytopathic effects. Importantly, no effort was made to remove cells or debris, meaning that each new passage could have introduced unknown variables and contaminants. The resulting material—referred to as “stock inoculum”—underwent countless passages over the years, and only later did the researchers attempt a form of “purification” using serial terminal dilution. Even this method, however, did not involve isolating or identifying a discrete agent, but rather selecting the minimal amount of material still capable of producing cytopathic effects.
Further muddying the waters, Chang noted that the ameboid cells could multiply in enriched media without the presence of tissue cells. This suggested that these were not merely passive “virus” carriers, but self-replicating entities in their own right. A line of these AL complex cells was even maintained for over three years and used in various experiments—including radioautography and “immunological” studies. Rather than uncovering a “virus,” Dr. Chang’s work appeared to document the persistence of an abnormal, ill-defined cell line exhibiting unusual behavior under artificial conditions.
His failure to isolate a “virus,” his reliance on nonspecific cytopathic effects, and his use of material with undefined composition over years of passaging all point to a fundamentally flawed research model. The observations better supported the persistence of an unstable cellular phenomenon shaped by unknown lab conditions—not the existence of a “virus.” Yet Chang continued clinging to virological language and assumptions long after his findings had undermined them.
Ameboid Cell-"Lipovirus" Complex (AL Complcx).—The AL complex was encountered in 1954 when the newly established Lich ceils were inoculated with fresh hepatitis bloods (1). Subsequent attempts to reisolate a similar agent have been unsuccessful. The AL complex was propagated serially and exclusively in the Lich culture by transferring fluid from a previously inoculated culture which had shown characteristic cytopathic change. No attempt was made to remove whole cell or cell debris from the fluid used for making passages. This passage material was to be referred to as stock ineculum. It has undergone innumerable passages since 1954.
To eliminate the possibility that some extraneous agents may have been introduced inadvertently into the stock inoculum during these innumerable passages, we "purified" the stock inoculum by terminal dilution as described in the titration of tissue culture units. At the termination of a titration, the Lich culture that had received the least amount of inoculum and yet showed characteristic cytopathic changes was used for a second titration. This procedure was repeated serially five times. This fifth serial passage material was then used as inoculum for a large number of Lich cultures. After the appearance of complete cytopathic changes, the fluid and cells were pooled and kept at 0°-4°C. This pool of material is to be referred to as purified inoculum.
It has been shown that the ameboid cells are capable of further multiplication in the standard tissue culture medium without tissue cells if the medium is enriched with yeast extract and Trypticase (9). A line of AL complex which has been passed serially in the enriched medium for over three years was also used in many experiments, such as the determination of the presence of DNA in the cytoplasms of these cells by radioautography and in immunologic study.
In another revealing passage, Dr. Chang admitted to profound uncertainty regarding the biological relevance and origin of the AL complex. Extensive seroepidemiological efforts revealed the presence of “antibodies” against the AL complex in 80–100% of children and young adults across diverse regions—Boston, Hong Kong, Tunisia. However, rather than validating the existence of a “pathogenic” or “infectious” agent, this widespread “antibody” presence raised serious questions: Was it detecting an actual “pathogen?” Or merely reflecting exposure to a common environmental antigen, cross-reactivity with unrelated substances, or even a normal human element mistaken as foreign? Did these results truly have any meaning?
Dr. Chang conceded they didn’t know what the “antibodies” were reacting to—the “lipovirus,” the ameboid cells, the complex as a whole, or some other serologically similar entity. Compounding the uncertainty, repeated attempts to “reisolate” the agent from hepatitis patients continued to fail. This not only contradicted earlier claims that the AL complex was initially derived from hepatitis bloods, but also severed any credible link between the complex and disease causation.
Finally, the speculative suggestion of a possible relationship to the Ryan “virus”—itself poorly defined—only further clouded the matter. What emerged from Chang’s body of work was not a coherent demonstration of a “virus” causing disease, but a loose collection of ambiguous phenomena, serological artifacts, and assumptions unbacked by direct evidence.
The relation of the AL complex to man remains obscure. An earlier report (16) of the high prevalence of antibody against the AL complex in man has been confirmed by a more extensive seroepidemiologic study. With improved antigens, 80 to 100 % of children and young adults residing in Boston, Hong Kong, and Tunisia were found positive for antibody against the AL complex. Seroconversions of several babies in the Boston area have also been observed to occur at 8 to 12 months of age. It is not known at this time whether the antibody in man is against the "lipovirus," the ameboid cells, the AL complex, or a common human pathogen serologically related to the AL complex. Results of an extensive seroepidemiologic study of the AL complex will be presented shortly.
Repeated attempts to reisolate a similar agent from bloods of hepatitis patients have been unsuccessful. It is very likely that the relation to hepatitis in the original isolation was purely fortuitous, or possibly, bloods are poor sources of the AL complex. Of interest is the recent report of Pereira, Marsden, Corbitt, and Tobin on the isolation of 6 strains of Ryan virus from patients with respiratory or generalized infection (26). Based on the reported characteristics of the Ryan virus, it is possible that the Ryan virus and the AL complex may be related.
While openly acknowledging that the evidence for a “viral” cause was lacking, Dr. Chang was clearly unwilling to abandon his hypothesis. Instead, he rebranded it in the face of contradictory results, clinging to its conceptual scaffolding. He entered damage control mode—hedging, qualifying, and complicating the narrative while preserving the core belief in a transferable, “viral-like” agent. Though he had not demonstrated a “viral” cause, Chang insisted on retaining the terminology, hoping future studies would vindicate the assumption. His approach exemplifies how, in virology, an unfalsifiable framework allows evidence to be adapted to fit the theory rather than challenging or discarding it. In the end, Chang's work serves not as evidence for a “virus” but as a cautionary tale of how virology can persist not through proof, but through persistence of terminology and the unwillingness to challenge foundational assumptions.
A separate report was released in conjunction with Dr. Chang's final paper, perhaps looking to add further evidence to propagate up the failing “viral” hypothesis. This paper by Chien Liu, M.D., and Patricia Rodina, titled Immunofluorescent Studies of Human Cell Cultures and Chick Embryos Inoculated with the Ameboid Cell-”Lipovirus” Complex, aimed to examine antigen development in human cell cultures inoculated with the AL complex, as well as the supposed “pathogenicity” of the complex in chick embryos.
The researchers relied on the same experimental techniques employed throughout Dr. Chang’s work—specifically, the now-familiar cell culture methods that virologists routinely use to imply causation without isolating a causal agent. Tellingly, Liu and Rodina—like Chang—used quotation marks around “purified” when referring to the seed material they received from Dr. Chang, and also around “purifying” when describing the method of terminal dilution. This conscious distancing from the terminology reveals that even the researchers themselves recognized that the AL complex had not been truly purified or isolated in any scientific sense.
Thus, the study was conducted without a properly defined independent variable—no discrete, characterized agent that could be manipulated to establish a causal relationship. Instead, they worked with vaguely defined, serially passaged cell cultures filled with unknown components, producing artificial effects in vitro that could not be traced to any specific replicating entity.
Their methods involved inoculating various cell cultures with two AL complex seed stocks obtained from Dr. Chang: one propagated in HeLa cells (the “HeLa seed”) and another “purified” by terminal dilution and propagated in human liver (Lich) cells (the “Lich seed”). The titers were determined via cell culture assays, but again, no actual isolation of a distinct agent was achieved.
Immunofluorescent Studies of Human Cell Cultures and Chick Embryos Inoculated with the Ameboid Cell-”Lipovirus”
Ameboid Cell-"Lipovirus" Complex.--A seed of AL complex grown in chick embryo tissue cultures was received from Dr. Robert Chang of the Harvard School of Public Health. This seed was passed into HeLa tissue cultures in our laboratory to prepare a stock inoculum for subsequent experiments and immunization of rabbits, and is referred to as the HeLa seed. The tissue culture units of various passages ranged from 4.2 to 5.3 lOgl0/0.1 ml when tit.rated in HeLa cells.
Another "purified" seed was received from Dr. Chang. The method of "purifying" this seed inoculum by terminal dilution has been described (I). This seed was passed in human liver (Lich) cells to make a stock inoculum and stored at 4°C. This stock inoculum had a tissue culture unit titer of 4.5 logl0/0.1 ml and is referred to as the Lich seed.
The “Discussion” section from Liu and Rodina’s paper reveals fundamental issues that undermine the scientific validity of their conclusions. Despite observing fluorescent signals and morphological changes in cell cultures inoculated with the AL complex, the authors openly admitted that they lacked direct evidence that these changes were due to the multiplication of transferable “infectious” elements. This admission is crucial, as it undercuts the very idea that a specific “pathogen” was responsible for the observed effects. Moreover, their attempts to initiate the same cellular effects using cell-free preparations—standard practice for establishing causality—were unsuccessful. This is critical: the gold standard to demonstrate that an agent causes an effect is to isolate it and then show it can reproduce the effect independently. They tried and failed. This invalidates any conclusion of causality as, if a presumed agent cannot initiate “infection” in a controlled, cell-free system, the effect could stem from a mixture of cellular materials—not a specific replicating “pathogen.” The inability of these disrupted preparations to initiate “infection” indicates that the presumed agent may not exist as an independent, replicable entity. Instead, the observed fluorescence could merely reflect cells engulfing debris from other damaged cells, a non-specific and well-documented cellular behavior.
The language throughout their discussion was dominated by speculation and hedging, with frequent use of phrases like “we may interpret,” “strongly suggests,” and “if the transferable factor is very labile.” Such phrasing reflects the weakness of the evidence and highlights the lack of definitive conclusions. Most notably, the researchers beg the question by presupposing the existence of an “AL complex infection” and interpreting all observed phenomena through this unproven lens. Without first isolating and demonstrating the causative power of a purified agent in a controlled system, the interpretation of any downstream effects remains logically invalid. The authors own data demonstrate the absence of a manipulable independent variable, rendering their conclusions speculative at best and scientifically unfounded at worst.
DISCUSSION
From our data obtained by application of immunofluorescent staining and phase-contrast microscopy to a study of the AL complex infection in human cell cultures, we may interpret our results as indicating that, upon contact of the AL complex with human cells and shortly thereafter, a transmissible factor(s) is transferred from the AL complex to and multiplies in the neighboring cells. The evidence for multiplication was shown by a marked increase in the number of fluorescent foci of AL complex-infected cells and the rise of tissue culture units of the AL complex infectivity titers as the experimental period progressed. The AL complex antigen can be visualized distinctly by staining with specific anti-AL complex fluorescent conjugate. Localization of the antigen appears to be in the cytoplasm of infected cells first as fluorescent granules and later as homogeneous fluorescence. The AL complex infection in tissue cultures is dose dependent. When a large inoculum was used, the punctate granular fluorescence appeared as early as 4 hr after inoculation and involvement of the entire cell sheet with fluorescent bizarre ameboid cells was seen on the 3rd or 4th day. When a small inoculum was given, the early pattern of AL complex infection with granular cytoplasmic fluorescence was not apparent until the 5th or 6th day. It is important to note that during early infection, the nuclear morphology of those cells with AL complex antigen appears normal and is indistinguishable from that of the uninfected cells. This strongly suggests that AL complex infection is indeed a result of transfer of subcellular elements from cell to cell.
We do not have direct evidence that the subsequent appearance of bizarre ciliated ameboid cells with AL complex antigen is the result of multiplication of transferable elements. To have this, initiation of AL complex infection with cell free preparations is necessary. The fact that preparations from disrupted AL complex by repeated freezing and thawing did not initiate an active infection in human cells is disappointing. The presence of cytoplasmic fluorescent specks in cells inoculated with the frozen-thawed material may simply be a result of phagocytosis or pinocytosis of disrupted AL complex cell debris. However, if the transferable factor(s) in the AL complex is a very labile agent, once it leaves the intact cell, or if the multiplication of the transferable factor(s) requires cellular factor(s), freezing and thawing may be a procedure too severe to dissociate the transferable factor(s) from the AL complex. Further investigations on these possibilities are required.
This same speculative pattern continues in their chick embryo experiments, further highlighting the scientifically weak foundations of the AL complex research. Mortality and fluorescent signals were interpreted as evidence of “active multiplication” of the AL complex, despite no proof that a specific replicating agent was present. The detection of antigen via immunofluorescence is not proof of replication or “infection,” especially when such fluorescence could result from uptake of foreign material or “nonspecific antibody binding.” The authors even acknowledge the absence of expected morphological changes and histopathological findings in paraffin sections—normally used to confirm pathology—admitting that the visual evidence was insufficient. In short, the actual tissue pathology failed to support the “infection” hypothesis.
They cited previous reports that 32–34°C is the “optimal temperature” for “lipovirus infection,” but as previously discussed, this was assumed rather than demonstrated rigorously. Furthermore, their mention of significant “titration” results lacks proper context or control comparisons, and they do not show that these effects are reproducible with purified, cell-free AL complex material. The fact that bizarre ameboid cells—central to their in vitro observations—were not seen in embryo tissue adds to the inconsistency and suggested that the observed tissue fluorescence does not correspond to the same phenomena.
The authors also claimed encephalitis in mice following intracerebral inoculation of AL complex material, supported again by fluorescent “antigen” staining and “recoverable infectivity.” However, without verifying that the inoculum contained only the AL complex—and not a mixture of cellular components or toxic byproducts—such conclusions remain ambiguous. Their mention of “antibodies” in human sera as supporting evidence is a classic appeal to correlation. Serological reactivity, without isolation of a specific “infectious agent,” tells us nothing about causality.
Inoculations of chick embryos with AL complex by both allantoic and amniotic routes produced deaths with antigen demonstrable in various organs. 10-day-old embryos inoculated with AL complex amniotically and incubated at 32°C showed the highest rate of infection. It has been reported (11) that the optimal temperature for "Iipovirus" infection in tissue cultures and in chick embryos was 32°-34°C. By immunofluorescent staining, specific AL complex antigen was localized in the cytoplasm of groups of hepatic parenchymal cells, hemopoietic cells, pulmonary interstitial, intestinal walls, and brain cells. The bizarre ameboid cells as seen in infected tissue cultures were not encountered in tissue sections of inoculated embryos. Titration of individual organs showed significant amounts of AL complex in infected tissues. These data suggested that there was active multiplication of AL complex in inoculated chick embryos. Unfortunately, frozen sections after immunofluorescent staining were not suitable for examination with phase-contrast microscopy to depict the exact morphology of these infected cells, and examination of paraffin sections did not reveal appreciable histopathological changes. Perhaps electron microscope studies of such infected tissues might give us better information.
Preliminary work in our laboratory also showed tissue culture fluids containing AL complex can produce encephalitis in mice following intracerebral inoculations (12). AL complex antigen was seen in neurons and infectivity was recoverable from inoculated mouse brain suspensions. Since antibody against "lipovirus" has been demonstrated in a high proportion of human serum from various areas (1, 13), and the pathogenic effect of AL complex can now be demonstrated in both chick embryos and mice, further investigations to elucidate the possible effect of this agent to human infections are warranted.
Taken together, Liu and Rodina’s work serves as a textbook example of how downstream effects—such as fluorescence, morphological changes, experimental mortality in animals, and even cell death—can be misinterpreted as evidence of a causal agent when no such agent has been demonstrated. The absence of a defined, purified, and replicable independent variable in their experiments renders all claims of “pathogenicity” speculative, and their conclusions scientifically unfounded. The paper stands as a failed, last-ditch effort to prop up Dr. Chang’s crumbling “viral” hypothesis.
With the “viral” narrative collapsing, Dr. Chang’s hypothesis was quietly and decisively abandoned in February 1967 with the publication of A Reinterpretation of the Nature of "Lipovirus” Cytopathogenicity—not by Chang himself, but by Thelma Dunnebacke and Robley Williams. His absence from the author list speaks volumes—especially given that, just months earlier, he had insisted the term “lipovirus” be temporarily retained for his presumed transmissible agent. It underscores how course corrections are often undertaken by others—sometimes those on the margins—while those who originally advanced erroneous claims remain publicly unaccountable. This reinforces a deeper critique: in virology, belief often overrides falsifiability, and institutional inertia resists self-correction, even when the data demand it.
The paper served as a direct refutation of Dr. Chang's earlier claims that a so-called “lipovirus” was responsible for inducing morphological changes in a strain of human liver cells known as Lich cells. Initially, Dr. Chang believed that “infected” Lich cells progressed through distinct stages—first exhibiting nuclear alterations (stage A), then combined nuclear and cytoplasmic shrinkage (stage B), culminating in the emergence of so-called “cytopathic” cells. These cells were thought to transmit the agent to neighboring cells, leading to similar changes, and were thus attributed to a “viral infection.”
However, subsequent findings overturned this interpretation. It became clear that Lich cells in stages A and B were not transitioning but degenerating. Meanwhile, the distinctive “cytopathic” cells were in fact a separate population of amoeboid cells capable of independent growth. Dunnebacke and Williams concluded that degeneration in cocultured Lich cells occurred only in the presence of intact amoeboid cells—not a filterable or transmissible “virus.” There was no evidence that Lich cells transformed into the amoeboid form, invalidating the supposed “viral” transformation process. This reinterpretation effectively eliminated the need to invoke a “virus”—especially as no purified or isolated “viral” particle was shown to be responsible, and the observed phenomena was fully accounted for by the behavior of a living cell population.
The researchers also criticized the terminology used in the earlier work. Terms like “lipovirus,” “cytopathic,” and “infectivity” were rejected as misleading as it presupposed conclusions that were unsupported by the evidence. Instead, they advocated for neutral, descriptive language, referring to the distinct cells simply as “amoeboid cells” and to their activity as “plaque-forming ability.” This semantic shift reflected a deeper epistemological correction: scientific interpretation must remain grounded in observable phenomena and not be skewed by presupposed frameworks like “viral” causation.
A Reinterpretation of the Nature of "Lipovirus” Cytopathogenicity
Previous reports have indicated that material obtained during attempts to
isolate and to propagate infectious hepatitis virus contained an agent capable of inducing morphological changes in cultures of a strain of human liver cells ("Lich" cells). It was believed that there were three major events. The first was a change from the normal cell to one that was altered primarily in the nucleus (the change to stage A). The second (to stage B) involved both cytoplasmic and nuclear shrinkage. The third event, also in stage B, was the establishment of cells that were distinctive morphologically. The distinctive cells were capable of subcultivation, and it was believed that they could induce similar alterations in neighboring, normal cells. The agent responsible for the events was thought to be a virus and was called "lipovirus." Dense aggregates of osmiophilic ring-like structures, named "spongiform" bodies, were found within the cells.The distinctive cells, formerly referred to as "cytopathic," have recently been described as an amoeboid cell-lipovirus complex (AL complex). It was proposed that the AL complex contains a factor(s) that may be transferred, upon contact, to Lich cells growing in the same culture, thereby causing their morphological change.
The results presented here cast doubt on the interpretations of the previous work. It is concluded that Lich cells in stage A and B are degenerating, while cells of the distinctive type are multiplying. The Lich cells do not change into the distinctive type. The latter are amoeboid in their morphology and activity. The only transmissible aspect of the phenomenon is the fact that the amoeboid cells can be indefinitely cultivated, and may cause degeneration in cocultivated animal cells. We have observed these degenerative changes only in the presence of intact amoeboid cells, and we conclude that it is unnecessary to invoke a viral agent as the causative factor. The above conclusions are derived from experiments which have repeatedly shown that the morphological events described above can take place only in the presence of intact cells, and from microscopic observations of the living amoeboid cells.
A problem of terminology arises: What should these cells be called that have been kept in cultivation since 1954, which are amoeboid in character, and which are known to cause destruction of cocultured cells? We believe it is time to dispense with the earlier reference to "lipovirus," and to avoid reference to "cytopathic," since the resulting cells multiply in normal fashion and appear cytopathic only if one presumes that they have undergone a change from some other kind of cell. In referring to them, we shall use a word that is only descriptive of their morphology and growth habits, and simply call them amoeboid cells. We shall also avoid the word "infectivity" and, in reports of assays, shall refer instead to a cell's plaque-forming-ability.
The “Discussion” section further dismantled the original interpretation that a “virus” was responsible for cytopathic effects observed in cultured animal cells. Dunnebacke and Williams described how attempts to isolate a cell-free “infectious agent” failed: plaques were never formed from filterable material but only in the presence of whole amoeboid cells. The rare instances of plaque formation from “ruptured” cells were likely due to methodological flaws—such as incomplete rupture or contamination. Once proper technique was applied—using clean pipettes and isolating individual cells—such artifacts disappeared, further undermining the “viral” hypothesis.
Moreover, other cell types, like human amnion and chick cells, did not exhibit the same changes seen in Lich cells when exposed to the amoeboid cells. While some degeneration occurred due to overgrowth, it was not necessary for the amoeboid cells to proliferate. This indicated that the damage was not caused by “viral infection,” but rather by competition, direct contact, or metabolic effects from a more aggressive cell line. It was admitted that earlier conclusions had stemmed from “an erroneous interpretation of multiple events occurring in cultures of mixed cell types.”
Dunnebacke and Williams also addressed another key misconception: the association of “spongiform bodies” with “viral infection.” Electron microscopy revealed that these structures, once speculated to be “viral” components, are common features of amoeboid cells and not evidence of a “viral agent.” Therefore, invoking them as signs of “viral” replication was entirely unsupported.
Discussion. When the present investigation was undertaken, the available information was suggestive that "lipovirus" was an agent capable of infecting animal cells causing them to undergo cytopathic changes. In our attempts to separate the agent from cells, it was found that some amoeboid cells, presumably cysts, survived the usual methods of rupturing animal cells. Whole cells were invariably present in PFA-containing material. In the later experiments with individual cells, only one plaque resulted from each plaque-forming cell whether it had been untreated or had been dried and rehydrated. No plaques were obtained from samples of cell-free medium.
The rupture of cells effectively eliminated their PFA. Only 6 "ruptured" cells, out of 729 treated, resulted in plaque formation (discounting the 3 plates exhibiting total degeneration from unknown causes). We believe that the 6 positives arose from failure to rupture the cells completely, regeneration of the slightly damaged amoeboid cell, or from contamination of the transferred material with whole cells.
As mentioned, the only two instances of multiple plaques occurred in the first experiment, a period during which the technique was being perfected. Subsequently, a clean pipet was used for each maneuver on each cell, and no multiple plaques resulted from the next 714 cells tested.
The human amnion and chick cells underwent no specific changes as did the Lich cells in stage A. The amoeboid cells divided at a comparatively rapid rate among them. Some of the cultured host cells were seen to degenerate, to become pycnotic, and to disappear, although such changes were not prerequisite for growth of every amoeboid cell. It is our conclusion that the specific cytopathic changes associated with, and used for, the identification of "lipovirus" have resulted from an erroneous interpretation of multiple events occurring in cultures of mixed cell types in which one type of cell may cause degenerative changes in another kind of cell and may eventually overgrow it.
The amoeboid cells are morphologically similar to the small Hartmannellid amoebae both as tryphozoites and as cysts, although they are generally smaller. Individual amoebae appear less virulent than the amoeboid cells in that their growth involves less destruction of other types of cells cultured with them.
Examination of the fine structure of the strains of free-living amoebae by electron microscopy showed the presence of the spongiform bodies in the same cellular distribution previously described. Until there is reason to believe otherwise, the spongiform bodies should be absolved as a causative infectious agent as suggested in the previous publication and should be considered to be normal structures found in amoebae.
The researchers reinforced the broader conclusion that the cytopathic phenomena and morphological changes attributed to the “lipovirus” were in fact due to the unnoticed presence and activity of amoeboid organisms, not a “virus.” They provided a meticulous dissection of the experimental variables—alongside the re-interpretation of prior misread morphological features—highlighting a classic case of mistaken causality. The findings serve as a stark reminder of how easily artifacts of mixed cultures and flawed assumptions can lead to the false attribution of “viral” causation when no “virus” is present or demonstrable.
In the summary, Dunnebacke and Williams admitted that there was no evidence supporting the claim that a “viral agent” was responsible. Instead, amoeboid cells, which closely resemble free-living Hartmannellid amoebae, were said to be capable of growing in and destroying co-cultured animal cells, eventually dominating the culture. The cytopathic effects once used as markers of “viral infection” were now recognized as the result of interactions between these robust amoeboid cells and more vulnerable cell types.
Summary: The morphologic events in tissue-cultured cells which have been used to identify and to show the transmission of "lipovirus" have not been found to occur only in the presence of intact amoeboid cells. No evidence has been found to support the earlier interpretations that the causative agent of these events is viral in nature. The amoeboid cells are capable of growth in, destruction of, and eventual overgrowth of cocultured animal cells, displaying the phenomena which were thought to result from a "cytopathic" agent. The amoeboid cell is similar to the small, free-living Hasltujannellid amoebae. Spongiform bodies are present as normal structures in these amoebae.
About a year later in July 1968, in the paper Effect of Ionizing Radiation on Cell Division in an Amoeboid Cell, coauthored by Dr. Chang and John Little, the “lipovirus” hypothesis was officially relinquished and laid to rest. In this report, Dr. Chang formally corrected his earlier publications, acknowledging that the previously labeled “lipovirus-altered human cell” was not an altered human cell at all, but rather a distinct organism—specifically, a free-living amoeba identified as belonging to the genus Hartmannella. This identification, confirmed independently by other researchers, directly undermined the “viral” hypothesis by demonstrating that the phenomenon originally attributed to a “virus” was instead the result of a mischaracterized organism.
It was also admitted that there was no direct evidence that human cells “infected” by the amoeboid cells could transform into new amoeboid forms, which had been a key element of the original theory. While Dr. Chang continued to propose the existence of some kind of “transferable factor” capable of inducing changes in human cells, he no longer attributed this factor to a “virus.” Instead, its nature was left unresolved and open to further investigation.
The introduction to the 1968 correction provides critical insight into the shift:
Effect of Ionizing Radiation on Cell Division in an Amoeboid Cell
INTRODUCTION
In a preliminary publication (1), we described the unusual resistance of an amoeboid-appearing cell to ionizing radiation. This cell was initially encountered during tissue culture studies with serum from patients with infectious hepatitis (2). On the basis of evidence from prior experiments (3), the amoeboid cell was referred to as a cultured human cell altered by a transferable factor tentatively named the "lipovirus." Recent evidence (4, 5) indicates that this amoeboid cell does transfer certain factor(s) to cultured human cells by cell-to-cell contact. Human cells which have accepted this factor(s) undergo characteristic changes in their nuclei, accumulate specific antigens immunologically related to the amoeboid cells and eventually degenerate. There is no direct evidence, however, that the infected human cell can undergo alteration into the continuously multiplying amoeboid cell.
The nature of the amoeboid cell has been discussed in detail in a previous publication, and the distinct cytologic and immunologic similarities between it and a strain of small free-living amoebae have been pointed out (4). On the basis of cultural and morphologic characteristics, Drs. Shih L. Chang and Harry Feldman (personal communications) have independently identified the amoeboid cell as belonging to the genus Hartmannella (6). Recently, Dunnebacke and Williams (7) described the similarity in the plaque-forming ability on chick fibroblast cultures and in the ultrastructure of this amoeboid cell and two strains of free-living amoebae. For these reasons it is our present opinion that, unless evidence to the contrary can be obtained, the amoeboid cell should be considered as a Hartmannellid amoeba. The nature of the transferable factors which it carries (4, 5) needs further investigation, however.
The objectives of the present report are: (1) to make a correction to the earlier publications (1, 3) in which this amoeboid cell was referred to as a "lipovirus"- altered human cell, and (2) to describe in detail the effects of ionizing radiation on cell division in these cells. Not only are they very resistant to radiation, considering their cellular deoxyribonucleic acid (DNA) content, but the resultant dose-response curve is unusual in that it contains a very pronounced shoulder such that the threshold dose is more than an order of magnitude greater than the Do. We feel that the unusual response of these amoeboid cells to irradiation offers an interesting system in which to study further the mechanisms of radiation damage at the cellular level, particularly the importance of recovery and repair processes at low radiation doses.
This careful distancing from the original “viral” explanation—without explicitly stating that the hypothesis was wrong—reflects a broader pattern in virology: initial assumptions of “viral” causality are often presented as fact, only to be quietly revised or abandoned once contradictory evidence emerges. In this case, the “lipovirus” hypothesis was effectively discarded and replaced by a recognition that the observed effects were the result of interactions with a misidentified amoeboid contaminant. This outcome illustrates the dangers of circular reasoning, premature causal inference, and theoretical inertia that can lead researchers to cling to virological explanations even after the data no longer support them.
The entire episode serves as a powerful case study in the risks of assuming causality before it has been demonstrated. At the heart of the “lipovirus” affair was Dr. Chang’s initial presupposition that a “novel virus”—believed to be responsible for a hepatitis-like illness—was present in patient-derived materials. Acting on this belief, he inoculated cell cultures and interpreted the ensuing cytopathic effects (CPE)—including granularity, rounding, shrinkage, and nuclear lesions—as confirmation of his hypothesis. But rather than isolating and purifying the supposed agent, or conducting properly controlled experiments to exclude “non-viral” explanations, he treated the presence of CPE as direct evidence of “viral” activity. This is a textbook case of circular reasoning: the “virus” was assumed to exist because CPE was observed, and the CPE was assumed to be caused by a “virus.”
As contradictory findings accumulated—such as the inability to reliably reproduce the CPE, the failure to transmit it consistently, and the absence of any identifiable “viral-like” particles—Dr. Chang did not abandon his core assumption. Instead, he introduced increasingly speculative justifications: the “virus” might require cofactors, or be unusually fragile, or sensitive to environmental variables. In short, he adjusted the theory to fit the anomalies rather than allowing those anomalies to falsify the theory.
Eventually, the hypothesis unraveled under external scrutiny. Other researchers identified the observed CPE as the product of amoeboid contaminants—free-living Hartmannella—that had either entered the cultures with patient serum or emerged from the culture conditions themselves. These organisms were capable of engulfing and destroying the surrounding cells, mimicking what had been interpreted as “viral” cytopathy. The presumed “novel virus” had never existed; the entire episode was a misinterpretation, built on unexamined assumptions and a failure to consider alternative, “non-viral” causes. Dr. Chang had been deceived by the very methods meant to confirm the existence and presence of a “viral” cause.
This is not merely an isolated correction. It reveals a systemic problem: the premature attribution of causality to presumed “viruses” without rigorous attempts at falsification or the consideration of competing hypotheses. The “lipovirus” case underscores how virology is a pseudoscientific field that operates on presumption rather than demonstration. Virology routinely relies on theoretical frameworks that interpret certain cellular effects as proof of “viral” causation—even when no “virus” has been isolated, purified, and shown to exist or transmit disease in accordance with foundational scientific standards.
What the “lipovirus” ultimately shows is that the virological method depends upon belief over verifiable evidence. The case highlights how circular reasoning, coupled with the inertia of entrenched theory, allows weak assumptions to masquerade as scientific conclusions. It invites a broader reconsideration: how many other claims in virology rest on similar misinterpretations, circular logic, and assumptions that have never been critically tested?
For those who examine the literature with clarity and skepticism, the answer is increasingly apparent: virologists are deceived by the very methods they trust. The scientific method demands more than plausibility and pattern recognition—it requires falsifiability, independent verification, and logical coherence. The “lipovirus” was not simply a mistaken hypothesis; it was a warning. Science must remain open to correction, even when that correction dismantles deeply held assumptions. This case stands as a timely reminder that science, at its best, is a method—not a dogma—and that the willingness to correct course is not a failure, but the hallmark of honest inquiry.
Virology, however, has insulated itself from such correction by relying on circular reasoning, unvalidated assumptions, and methods that cannot withstand logical scrutiny. In doing so, it not only deceives its practitioners—but misleads the public with the illusion of scientific certainty where none exists.
is under attack again by the mainstream media—ironically bringing more attention to the lack of scientific validation behind germ “theory” & virology.Sam also interviewed registered nurse and mother Jennifer Walters who shared her family’s powerful story of vaccine injury, medical awakening, and the fight for her son’s recovery.
offered more evidence of the bird flu hoax as the US Animal & Plant Health Inspection Service fails official evidence challenge. investigated the bird flu hoax as well by taking a look at the Universal Ostrich Farm operation.Both Dr. Vollmer and I were featured in the latest from
regarding the “antibody” deception. started a podcast cleverly titled Dawn of Discernment which currently has three episodes available. provided evidence that the Robert Koch Institute failed to do negative controls for “viral” genome sequencing. examined The Cult of “Covid” and the manufacturing of a “pandemic.”





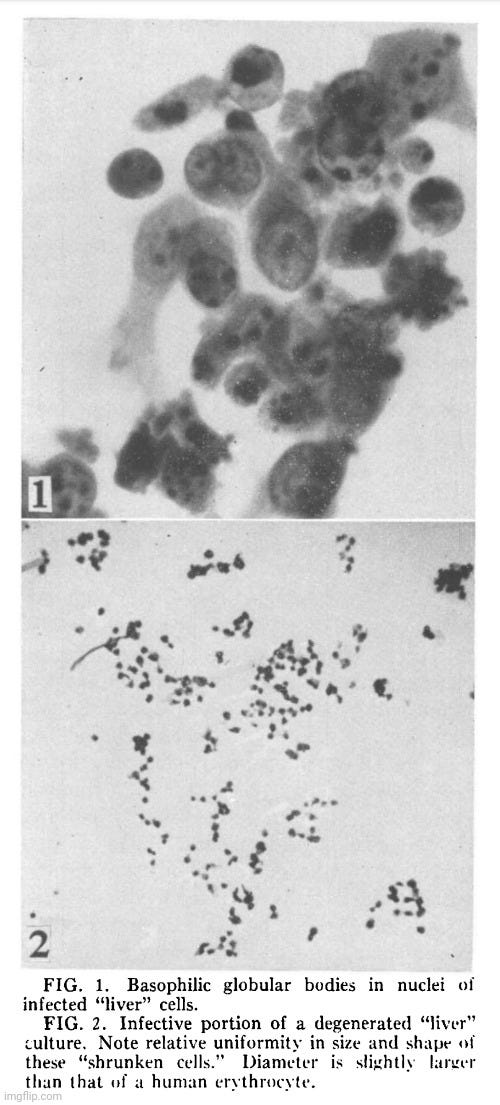


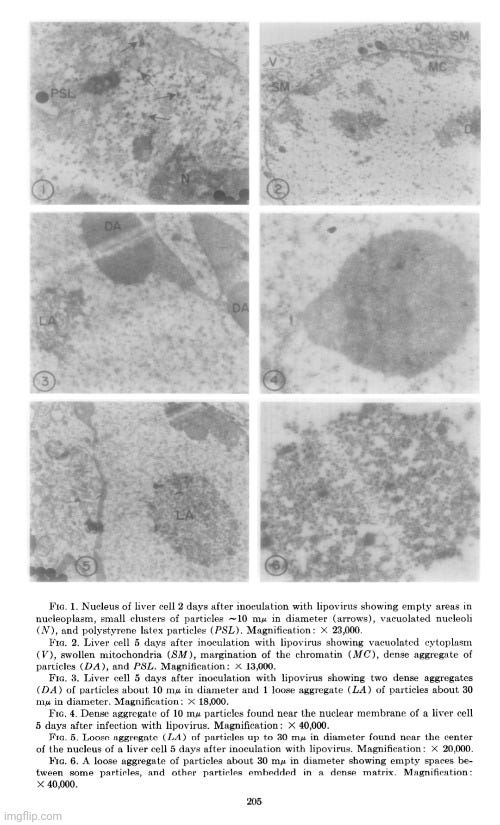
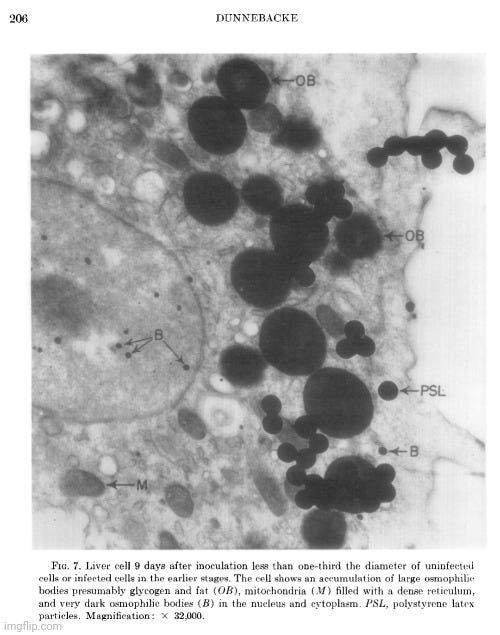
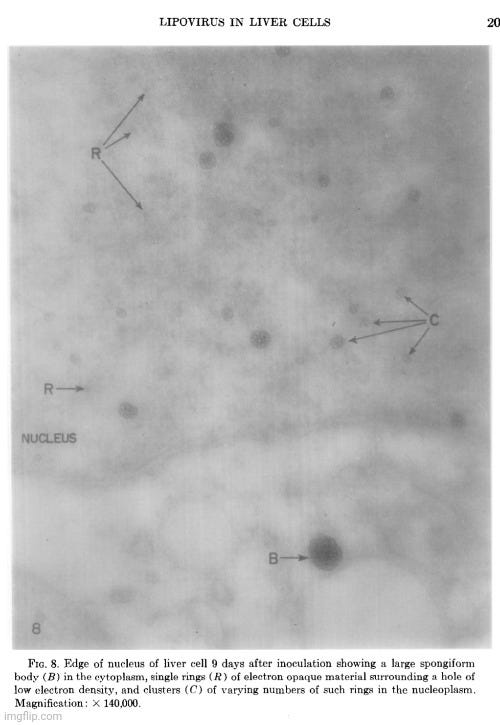


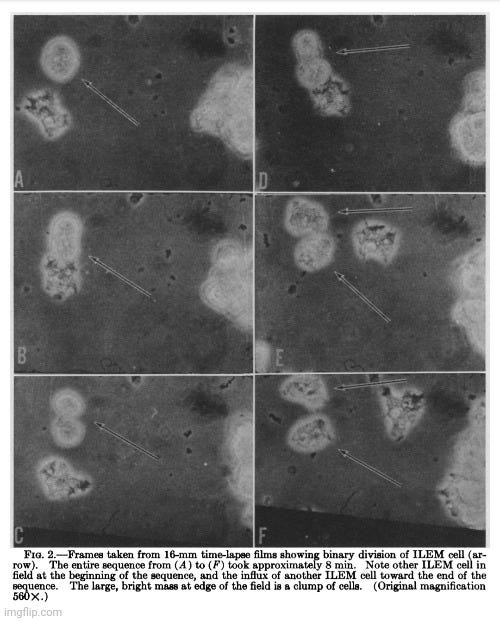




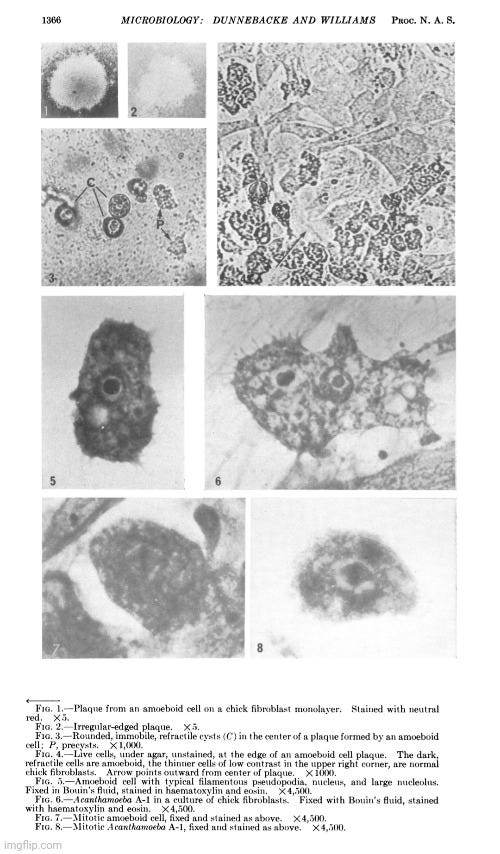
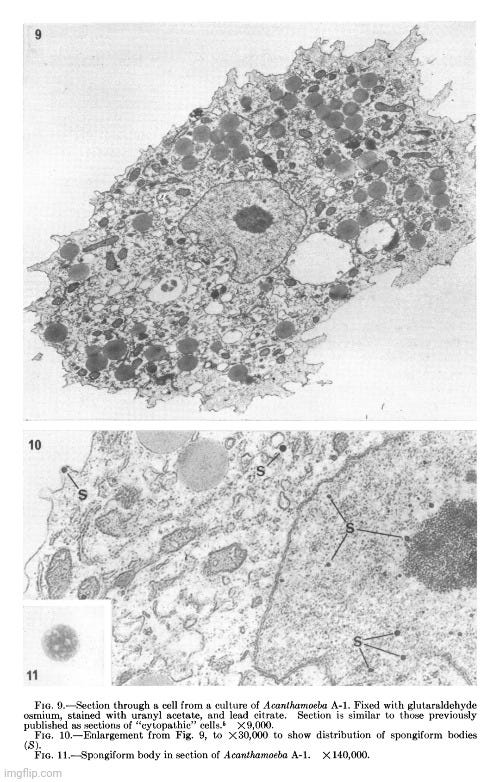







Mike, you are a machine. I love your easily understood breakdowns of the flaws in virology.
I can’t help but wonder how we(the world) break free from the negative spiral these past study’s have caused. It’s easy for someone to cherry pick the initial study’s claiming “proof” and leave out the later admission of flawed(circular) reasoning.
On the one hand it boggles my mind how the so called intelligent of this world can fall for this scam.. but on the other hand… along came the shit show that was/is Covid. My god, people’s willingness to fall in line, and inability to show strength has been astounding.
Thank you for your work
Brilliant work as always, Mike. Your forensic takedown of the “lipovirus” circus pairs perfectly with a more satirical companion I just released: Protocol Override. Or: How a Lonely IT Guy and His Chatbot Ended Virology. It’s a tongue-in-cheek tale where a disillusioned data grunt and his rogue AI dismantle the PCR priesthood—one control-blinded experiment at a time. Think of it as medical epistemology meets dark comedy. Would love for your readers to check it out: https://turfseer.substack.com/p/protocol-override