Science is the systematic study of the natural world. As outlined in Chapter 2 of Environmental Science, its fundamental goal is “to understand natural phenomena and to explain how they may be changing over time.” To achieve this, science depends on empirical observation, logical reasoning, and controlled experimentation—most often guided by inductive reasoning.
The systematic process utilized to acquire scientific knowledge is known as the scientific method, and it begins with the observation of natural phenomena: events or processes that occur without human intervention and can be detected by the senses. From these observations, scientists form hypotheses—falsifiable and testable explanations that define a presumed cause (independent variable) and predict an effect (dependent variable). The National Academy of Sciences defines science as “the use of evidence to construct testable explanations and predictions of natural phenomena,” emphasizing that all scientific inquiry must be grounded in the observable world. Any explanation that arises from phenomena not observed in nature—such as effects manufactured in laboratory conditions—cannot be tested, verified, or falsified, and therefore falls outside the bounds of science.
This distinction is critical when evaluating virology methods. The natural phenomenon under investigation—disease in a living host—is not being studied through direct observation or controlled replication of that condition. Instead, virologists rely on artificial methods that bear no resemblance to the phenomenon itself. Rather than purifying and isolating presumed “viral” particles directly from the fluids of a sick individual and testing their “pathogenicity” through natural exposure to a healthy host—as would befit the study of disease and the proposed route of transmission—virologists conduct complex cell culture experiments. In these procedures, an unpurified patient sample is added to a culture of cells—typically derived from animal kidneys or human cancer lines—and maintained for days in the presence of fetal bovine serum, antibiotics, antifungals, and other foreign substances. If visible cellular damage or death occurs, this effect—called the cytopathic or cytopathogenic effect (CPE)—is interpreted as indirect evidence of a “virus.”
However, the cytopathogenic effect is not a naturally occurring phenomenon, but rather an artifact of the laboratory setup. It arises from removing cells from their natural environment and exposing them to artificial and often stressful conditions, including toxic chemicals, foreign sera, and other unnatural additives. These factors alone are sufficient to cause cellular deterioration or death, regardless of any presumed “viral” presence.
This was acknowledged by John Franklin Enders, the man who brought this method to prominence in the mid-1950s. In a revealing 1954 paper titled Cytopathology of Virus Infections: Particular Reference to Tissue Culture Studies—published the same year as his influential measles study that showed CPE in the “uninfected” cultures—John Enders admitted that CPE could be triggered by many agents aside from “viruses.” He acknowledged that cytopathic effects are influenced by numerous factors, both known and unknown. These include the age and type of donor tissue, the culture conditions, and environmental variables. In other words, the cellular changes virologists attribute to a “virus” may have no connection to one at all.
“The phenomena mentioned above under Group 1 changes may be evoked by many noxious agents. Accordingly, they cannot alone be considered as necessarily the result of viral activity. To prove this certain control procedures (serial cultivation, prevention of changes by homologous antibody, etc.) must be applied. Familiarity, however, with the effects of a specific virus in a given cell system often enables the observer to conclude tentatively that this virus is responsible.”
“Of morphological indices of viral injury, the formation of inclusion bodies (Group 2 above) is the most characteristic, although again this process cannot be accepted as conclusive evidence of viral activity since certain chemical as well as other unknown factors may condition its development. Inclusion bodies were the first cytopathic changes to be sought for in vitro and employed as criteria of infection. As indices of viral multiplication, however, they are less useful than the changes of Group 1, because these structures can be unmistakably demonstrated only in stained preparations.”
“Cytopathogenicity in vitro is influenced by factors some of which are known while many remain to be defined. At the outset a few of those now recognized will be mentioned as an introduction to the review of recorded observations on the behavior of individual agents. Of primary importance is the species from which the cells are derived. Analogous to the host range of a virus is its cytopathogenic range in cultivated cells. But correlation between susceptibility of the organism and its cells in vivo does not always exist. For although this correlation frequently obtains, the tissues of a susceptible species occasionally fail to support viral multiplication while the converse of this situation also occurs.
The age of the donor of tissue may influence cytopathogenicity. Just as young animals are frequently more susceptible to infection so their tissues may be more vulnerable to injury by the virus, yet again this correlation is not invariable. Most of the pertinent data indicate that acquired immunity to viral infections is not reflected by an increased cellular resistance, a fact advantageous from the technical point of view since it eliminates concern over the immunologic status of the donor animal.
The intensity and degree of cytopathic injury may vary according to the strain of virus or the conditions under which it has been propagated prior to its study in tissue culture. The investigator should be prepared to encounter such variations in the study of a number of representatives of a viral species. Moderate or weak cytopathogenicity may sometimes be enhanced by serial passage in vitro.”
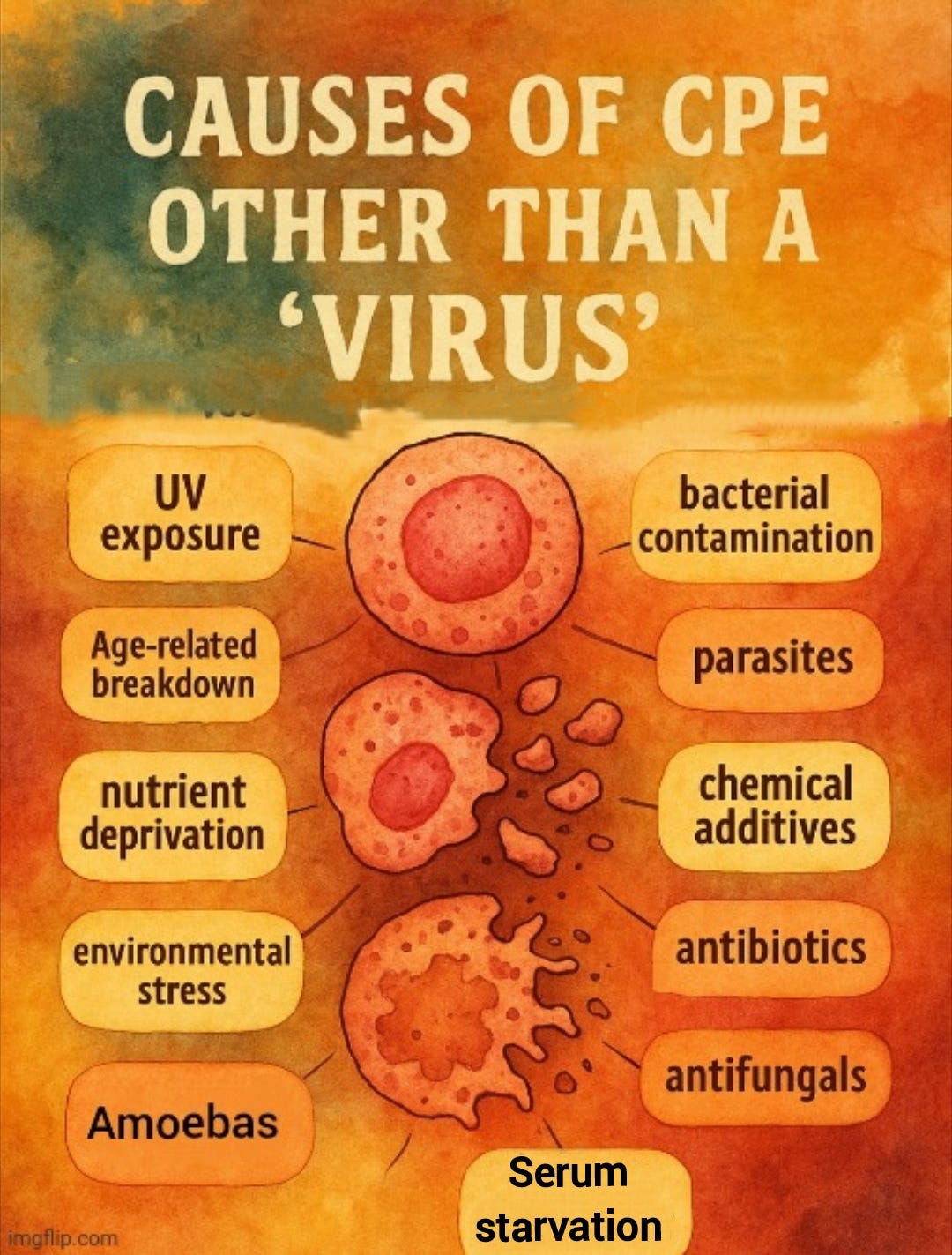
In fact, a variety of “non-viral” factors have been shown to induce the same CPE in cell cultures. These include bacterial contamination, parasites, amoebas, chemical additives, antibiotics, antifungals, nutrient deprivation, environmental stress, and even the age-related breakdown of the cells themselves. I have explored these factors in more detail in previous articles [here, here, and here].
Other notable factors that have been blamed for causing CPE in cell cultures include fibrinolytic agents, drugs, placental proteins, “virus-free” fecal samples that produced “profound” CPE resembling that attributed to “enteroviruses,” “immune” cells and “antibodies,” and the spontaneous degeneration of “virus-free” 12-week-old human embryonic tissue that mimicked the effects associated with the “cytomegalovirus.”
Even serum starvation—a routine step in virology protocols—has been repeatedly shown to induce cell death and morphological changes indistinguishable from the so-called CPE used to infer “viral” presence. This has been demonstrated in published studies and confirmed by Dr. Stefan Lanka in his independent investigations. Prolonged serum deprivation alone can lead to cell rounding, detachment, and apoptosis, meaning that the observed effects in cell cultures could result from the artificial conditions themselves, rather than from any “virus.”
For example, a 1997 study on NIH-3T3 cells found a loss of viability and cell death due to serum starvation and antibiotic use:
Antiapoptotic effect of ras in the apoptosis induced by serum deprivation and exposure to actinomycin D
“The present study reveals that untransformed NIH-3T3 cells respond with a loss of viability to serum deprivation and also to the cytostatic drug actinomycin D. The loss of viability is associated with the appearance of cells showing the classical features of apoptosis (nuclear condensation, cell shrinkage).”
“NIH 3T3 cells will remain in the cell cycle in the presence of serum growth support, whereas in the absence of the serum growth support the cycle is arrested in G1 and, after a period of several hours, the cells undergo apoptosis. Constitutive expression of v-H-ras turns on important signals to stimulate simultaneously G1 progression and repress cell death during serum deprivation.”
https://pubmed.ncbi.nlm.nih.gov/9050009/
Similarly, a 1999 study on V79 cells noted cell death and detachment from both serum starvation as well as antibiotic use:
Effect of serum starvation on expression and phosphorylation of PKC-alpha and p53 in V79 cells: implications for cell death
“The effect of serum starvation on the expression and phosphorylation of PKC-alpha and p53 in Chinese hamster V79 cells was investigated. Serum starvation led to growth arrest, rounding up of cells and the appearance of new PKC-alpha and p53 bands on Western blots. Prolonged incubation (> or = 48 hr) in serum-deprived medium led to cell detachment and death. Moving cells to fresh medium containing 10% serum before, but not after, cell detachment reversed the changes observed in PKC-alpha and p53, and also prevented later cell detachment.”
“Our observation of cell death induced by prolonged serum starvation or by exposure to staurosporine or actinomycin D is in agreement with other studies. Serum starvation (Chou and Yung, 1997), staurosporine (Couldwell et al., 1994) and actinomycin D (Chou and Yung, 1997) have all been shown to induce cell death via apoptosis.”
https://pubmed.ncbi.nlm.nih.gov/9935181/
These studies demonstrate that serum deprivation alone can cause the same cellular damage attributed to “viral infection.” As noted earlier, various other variables—such as bacterial contamination or chemical exposure—can also produce this damage, none of which require the existence of a “viral” agent. This highlights a critical flaw in virology: the reliance on effects that are not specific to a “virus” as supposed evidence of its presence.
Cytopathogenic effects are manufactured outcomes of laboratory manipulation, not observations of naturally occurring disease. Because CPE does not arise in nature and can result from a wide range of unrelated causes, it fails to meet the criteria for a valid dependent variable in experiments seeking to explain natural disease processes. Its use as evidence for “viral” presence and causation reflects a departure from empirical science—substituting artificial artifacts and speculative inference in place of observed natural phenomena and testable, falsifiable hypotheses.
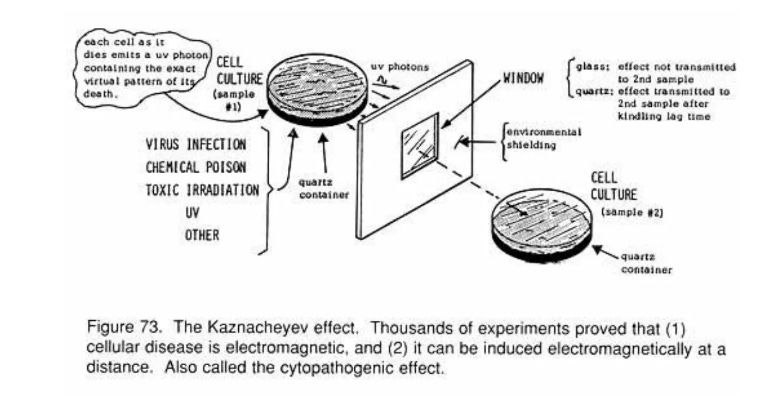
It is clear to those who understand natural science, the scientific method, and logical reasoning that cell cultures are a pseudoscientific setup. The literature is already rich with examples exposing the inherent flaws in this methodology. However, it never hurts to add more evidence—especially when it comes directly from the published record. One particularly revealing case that further exposes this scientifically invalid practice involves what became known as the mirror cytopathic effect (mCPE), where cellular damage was observed even when no presumed “viral” material was introduced. Thanks to the work of Vlail Petrovich Kaznacheev and colleagues in Russia during the late 1960s to early 1980s, we can drive yet another nail into the pseudoscientific coffin of virology by highlighting the nonspecific nature of its artificial, lab-created effects.
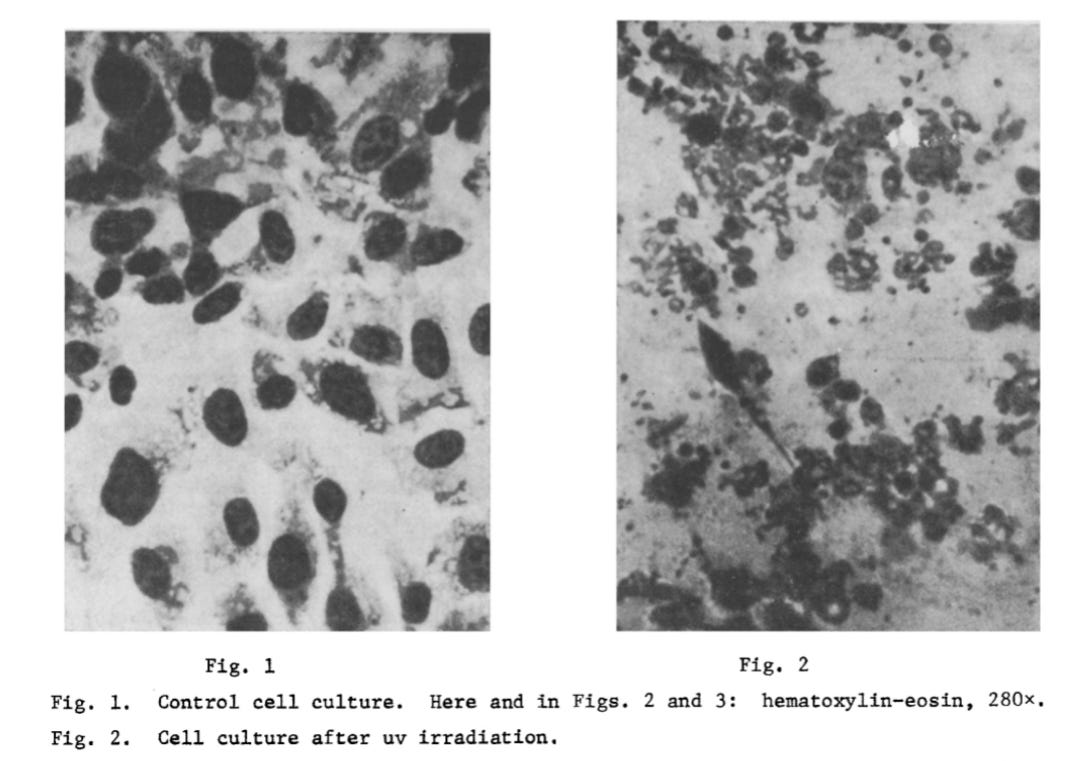
From the 1960s to the 1980s, Vlail Petrovich Kaznacheev—one of Russia’s leading medical scientists and founder of key research institutes—led numerous experiments to explore “non-contact” cell-to-cell communication. In his 1979 paper Conditions controlling the development of distant intercellular interactions during ultraviolet radiation, Kaznacheev reported that as early as 1966, his team had observed that cultured cells (“detector” cultures), grown on quartz supports, could mirror the radiation-induced changes of nearby cultures exposed to extreme chemical or biological stress. This phenomenon was termed the “mirror” cytopathic effect (mCPE).
To investigate this “non-physical” communication, Kaznacheev’s team designed experiments focusing on the role of ultraviolet (UV) radiation. Their 1979 studies followed two main paths:
Reproduction of the CPE in a mirror detector culture exposed optically (but not physically) to a UV-irradiated radiating culture.
Investigation of mCPE in detector cultures pre-treated with minimal UV, then placed in optical contact with “virus-infected” radiating cultures.
Using Hep-2 (human laryngeal carcinoma) and FECh (human embryonic fibroblast) cells, separated by either quartz (which transmits UV) or glass (which blocks it), they found CPE occurred in 384 of 500 experiments—but only when quartz was used. No CPE occurred through glass. In the second set of experiments, mild UV pre-treatment of the mirror cultures made it more susceptible to CPE when optically exposed to “virus-infected” cells—despite no “viral” material crossing between chambers. This demonstrated that CPE could arise from light-mediated, “non-viral” interactions, not “infection.”
Kaznacheev concluded that stressed or dying cells—especially those under UV stress—emit signals that can trigger specific morphological degeneration in nearby cells, even in the absence of direct contact or “viral” material. This challenged the conventional assumption that CPE is exclusive proof of “viral infection.”
“The objects of the investigation were to study the role of uv radiation in distant intercellular interactions (DII) and the conditions for obtaining a “mirror” cytopathic effect (MCPE). An extremal state of the cells in the radiating culture caused by uv-radiation was shown to induce a distant cytopathic effect (CPE) in an intact detector culture in optical contact only with it, reflecting the specific character of the morphological features recorded in the affected culture. Preliminary uv-irradiation of the detector cells facilitates manifestation of the MCPE.”
In 1980, Kaznacheev’s group expanded their work to explore whether electromagnetic radiation—specifically in the UV spectrum—could serve a biological signaling function. They exposed tissue cultures to stressors such as “viruses” (Coxsackie A-13 and fowl-pest “virus”) or mercuric chloride. Tissue cultures were chosen for their sensitivity and observable responses to cellular stress. When paired with healthy detector cultures—physically isolated but optically connected—the researchers found the same characteristic degeneration occurring in the mirror cultures.
Distant intercellular electromagnetic interaction between two tissue cultures
The existence of very weak intrinsic emission of radiation from biological objects (biochemiluminescence) is now generally accepted [1-4]. So far there have been few investigations aimed at determining the possible role of electromagnetic radiation in biological systems, although the possibility that biological objects can emit intrinsic radiation of different ranges has been demonstrated [5-7]. There is reason to suppose that electromagnetic interaction is a general principle of interchange of information among biological systems. Quanta with different frequency characteristics may perhaps be carriers of information.
Since 1966 the authors have studied the phenomenon of distant intercellular interaction due to electromagnetic radiation in the UV band [8-11]. The method of biological detection suggested by A. G. Gurvich has been used in our investigations to study the biological action of electromagnetic radiation in the biosystem.
Since we were interested to discover whether the electromagnetic radiation of cells performs a signal function, it was necessary to choose a state of the cells which could be clearly analyzed with the aid of the biological detector. A suitable object from this standpoint was a tissue culture infected with different viruses (Coxsackie A-13, the classical fowl pest virus -- FPV) or treated with mercuric chloride. In these cases the specific action of the viruses and mercuric chloride could be analyzed on the basis of their cytopathic action and immunologic changes. The experiments were planned so that the tissue culture infected with viruses or injured with mercuric chloride was the source of a specific signal, encoded in very weak radiation of the cells, and the intact tissue culture (not infected with virus) would serve as detector of this radiation. In the cells of the intact culture (henceforward designated the "mirror" tissue culture), in optical contact with the affected tissue culture, all morphological features of the extremal states specifically characteristic of the corresponding agent, developed. These morphological features are henceforward described as the "mirror" cytopathic effect (CPE).
The team used primary human and chick embryonic fibroblast cultures, as well as transplantable monkey kidney tissue cultures. In each experiment, both chambers were equally bathed in nutrient medium to ensure the cells remained nourished and viable. Controls were included to detect any spontaneous degeneration. Altogether, they conducted around 1,500 experiments with controls, carefully examining each for morphological changes.
EXPERIMENTAL METHOD
The tissue culture serving as the test object was grown in special chambers on quartz or glass slide supports of different thickness (from 0.2 to 2 mm), soldered to a ground-glass joint. The transmitting capacity of the quartz slides in the region of 280-320 nm was 70-90%. The maximum of transmitting capacity of the glass slides lay in the visible region, starting from 440 nm. Primary cultures of human and chick embryonic fibroblasts and also transplantable monkey kidney tissue cultures were used.
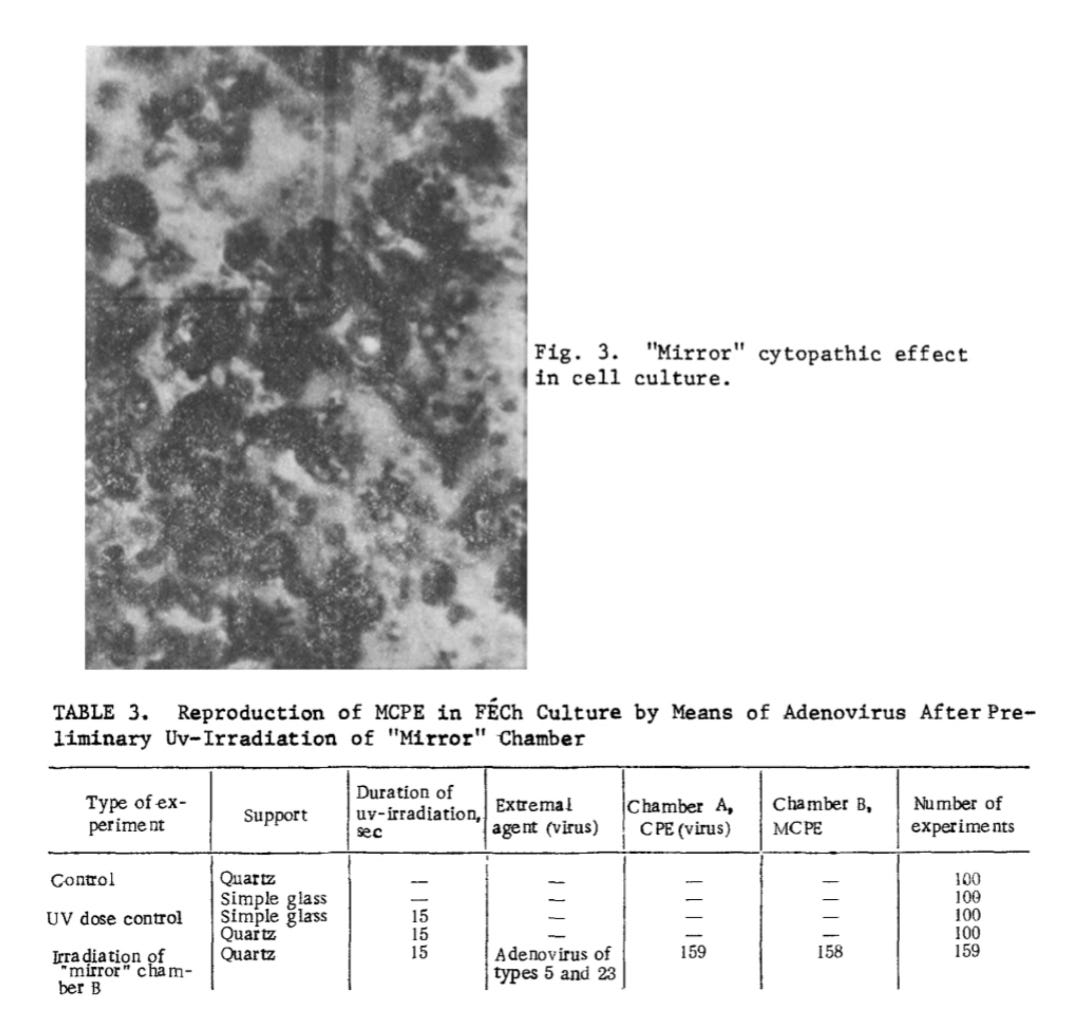
After a monolayer had formed on the floor of the chambers, the chambers with the introduced harmful factor were mounted in pairs with the intact slide supports and fixed to a revolving drum perpendicularly to the axis. The drum was placed inside a darkened thermostat (37°) and rotated together with the chambers at a speed of 25 rpm. The cells in the two chambers were thus bathed equally with nutrient medium, did not dry, and were adequately nourished. All experiments were accompanied by a control for detection of spontaneous degeneration of the tissue culture cells. After 2-4 days the chambers were removed and dismantled, the slide supports with cells growing on them were sealed off, and after fixation and staining, the cultures were examined morphologically. The CPE was calculated from the ratio of the number of dying cells to the total number of cells and from the type of morphological changes. The CPE was taken to be weakly positive if the ratio was 1:10, average if it was 1:5, and strongly positive at 1:2. Altogether about 1500 experiments, together with controls, were carried out.
In 350 experiments using the presumed Coxsackie A-13 “virus,” identical cytopathogenic effects occurred—but degeneration in the uninoculated “mirror” chambers consistently lagged 12–14 hours behind the “infected” cultures.
Interestingly, in typical virology experiments, researchers often stop incubation once only the “infected” culture shows CPE, interpreting this as evidence of “viral” activity. Yet in this case, even the uninoculated mirror chambers—essentially functioning as “mock-infected” controls—developed identical CPE, just slightly delayed. This seriously undermines the claim that earlier degeneration in inoculated cultures is evidence of “viral” causation.
Each experiment included controls to detect spontaneous degeneration in the “uninfected” chambers. Notably, when a simple glass barrier was used between chambers, no mirror CPE occurred. The presumed “A-13 virus” was regularly “isolated” from the “infected” chambers, but never from the mirror chambers—regardless of whether mirror CPE was present. A positive mirror CPE was observed in 74% of the experiments.
EXPERIMENTAL RESULTS
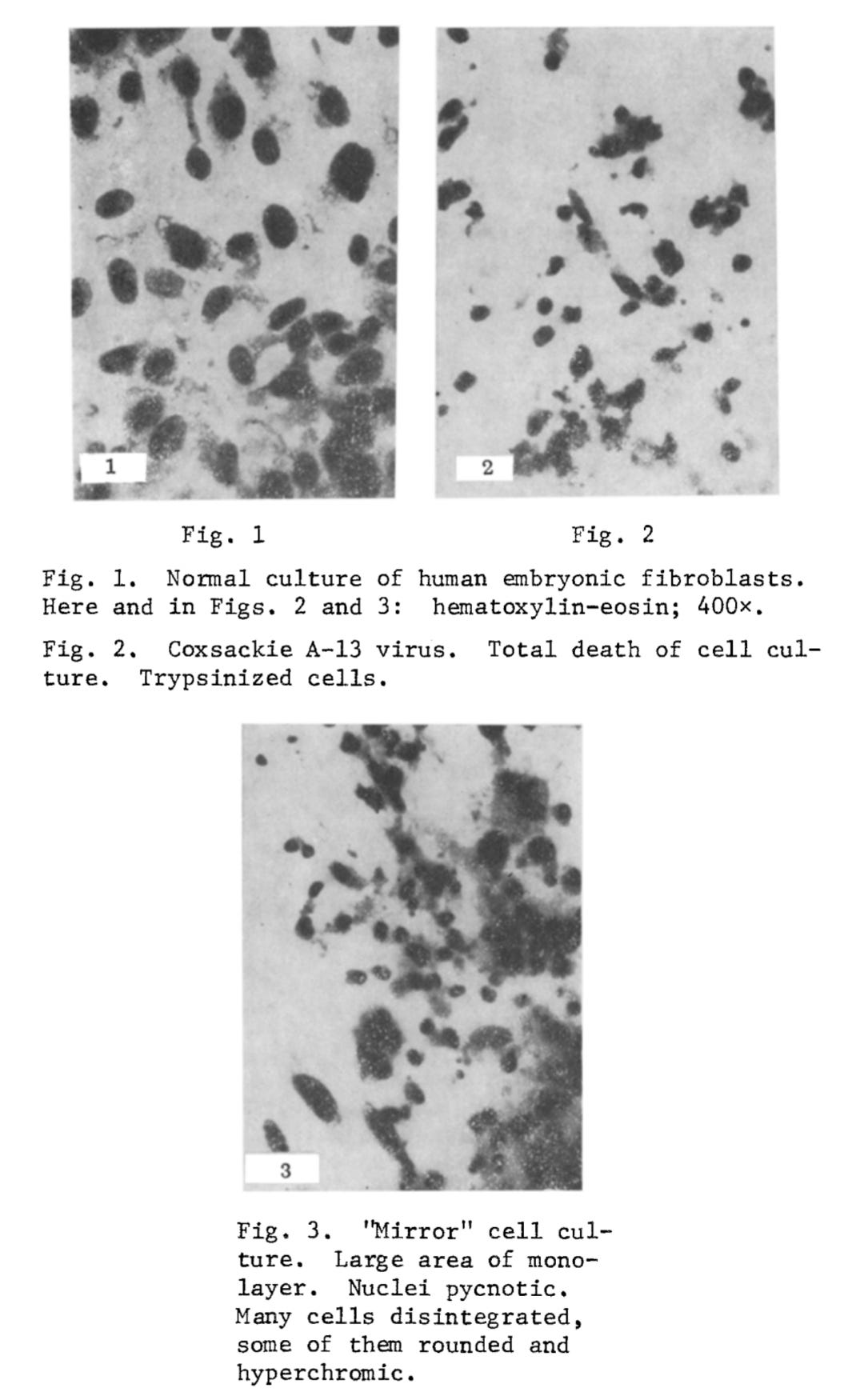
I. The "Mirror" CPE after Infection with Coxsackie A-13 Virus (350 Experiments). The cytopathic action of Coxsackie A-13 virus consists of breaking up of the monolayer and the appearance of round cells. Later the round basophilic cells undergo pycnosis: They shrink, become polygonal, and their nucleus becomes strongly hyperchromic. The pycnotic cells then disintegrate, and pycnotic "fragments" can be observed along with solitary intact cells. In the "mirror" cell cultures, inhibition of mitosis could be seen. The monolayer also was broken up with the appearance of pycnotic hyperchromic cells. Cells of this type later disintegrated, and in the "mirror" cell cultures, inhibition of mitosis could be seen. Cells of this type later disintegrated, and in the "mirror" cultures essentially the same evolutive forms of degeneration were observed as in cultures infected with virus. The tempo of development of degeneration in the "mirror" chambers was about 12-14 h behind. All experiments were accompanied by an appropriate control for detection of spontaneous degeneration in the uninfected culture. In chambers in which simple glass was used as the slide support, no "mirror" CPE developed. During passages from the infected chambers, A-13 virus was regularly isolated. Even after repeated passages, no virus could be isolated from the "mirror" chambers, whether with a positive or with a negative "mirror" CPE. A positive "mirror" CPE occurred in 74% of cases (Figs. 1-3).
In 453 experiments using the presumed fowl-pest “virus,” a “mirror” cytopathic effect (mCPE) was observed in 78% of cases. As with previous trials, only the “infected” chambers tested positive for the “virus” when their culture fluid was passaged. Negative results from indirect hemagglutination tests were taken by the team as “confirmation” that—even when a “mirror” CPE was present—“virus” was absent from the “mirror” chambers.
2. "Mirror" CPE after Infection with FPV Virus (453 Experiments). The experiment with FPV followed the same scheme as that with Coxsackie A-13 virus. In all the infected chambers degeneration characteristic of classical FPV was observed. It took the form of breaking up of the monolayer, rounding of many cells, and a tendency for the cells to form clusters. At the same time large, symplasmatic structures were formed, in the peripheral zone of which the nuclei formed a palisade. Later individual cells became shrunken. In uninfected "mirror" chambers in optical contact with the infected chambers, the presence of a "mirror" CPE was observed in 78% of cases. In the "mirror" culture many single rounded basophilic cells appeared, many of which later did not lose contact with each other, so that the monolayer became divided into a series of large cell complexes resembling bunches of grapes. The cytoplasm of the cells in these complexes was highly vacuolated and their nucleus was condensed.
Passage of the culture fluid from infected and noninfected chambers showed virus to be present only in the infected chambers. The hemagglutination test with fowl erythrocytes and culture fluid from chambers infected with FPV revealed hemagglutinins in the infected chambers in titers of 1:40, 1:80, and 1:160. Culture fluid from the uninfected chambers, even if a "mirror" CPE was present, did not give a positive hemagglutination test, evidence of absence of the virus.
In another 412 experiments, toxic injury was induced in chick and human fibroblast cultures using mercuric chloride as a “non-viral” model of cytopathic states. A toxic dose of HgCl₂ caused cell death within 2–3 days. When the experiment was performed under the same conditions as those with presumed “viruses,” a “mirror” CPE appeared in 78% of cases. This effect was statistically reliable, falling within 90–65% confidence limits at a 95% significance level. The frequency of “mirror” CPE caused by HgCl₂ was not significantly different from that seen with the two “viruses.”
3. "Mirror" CPE after Poisoning Cells with Mercuric Chloride (412 Experiments). To study the universality of the type of intercellular connections thus revealed, toxic injury to cells of tissue cultures of chick and human fibroblasts with mercuric chloride was chosen as a different model of cytopathic states. A toxic dose of HgCI2 was used, causing death of the tissue culture cells after 2-3 days through blockade of respiratory enzymes. In the chambers with HgCI2 a cytopathic effect developed with disintegration of the monolayer and granular and vacuolar degeneration of the cells and karyopycnosis. The process ended with total death of the monolayer. The "mirror" CPE also consisted of disintegration of the monolayer, vacuolation of the cytoplasm and karyopycnosis (78% of positive experiments). The achievement of a "mirror" CPE in the experiments with HgCI2 required the experiment to be performed under the same conditions as when viruses were used.
A control was set up for detection of spontaneous degeneration. In the chambers in which simple glass was used as the slide support, no "mirror" CPE of HgCI2 developed. Statistical analysis of the results pursued two purposes: I) determination of the probability of obtaining a "mirror" CPE for 95% confidence limits (5% level of significance of Pearson's criterion), 2) determination of similarity or difference between the action of viruses and HgCI2 by comparison of alternative distributions using Pearson's criterion.
The results showed that a "mirror" CPE can be reliably found within confidence limits of 90 to 65% and at a 95% level of significance. The effectiveness of action of the two viruses and of HgCI2 did not differ significantly as regards the production of a "mirror" CPE.
The experiments thus showed that in the presence of optimal contact between two isolated tissue cultures distant interaction takes place and is expressed as the repetition of the morphological features of the cytopathological process induced in one of the cultures by means of viruses or mercuric chloride, in the other intact tissue culture, or in other words, a "mirror" CPE takes place.
https://link.springer.com/article/10.1007/BF00834249
The work of Vlail Petrovich Kaznacheev and his colleagues is highly controversial, as it suggests that diseases may be transmitted electromagnetically—and, crucially, that no presumed “viruses” are necessary to elicit cytopathogenic effects (CPE). The implications are profound: in the “mirror” chamber experiments, CPE could not be attributed to “viral replication,” as no presumed “viral” particles were introduced, and none could be “isolated” afterward. The only consistent variable distinguishing setups that produced mirror CPE from those that didn’t was the transmission of ultraviolet light.
This directly challenges a core virological assumption: that CPE is a reliable indicator of “viral” activity. If identical cell degeneration occurs in the complete absence of any supposed “viral” material, then CPE cannot be considered definitive evidence of “infection” or “viral” presence. That the mirror cultures displayed the same degenerative patterns—with only a time delay—suggests the presence of a causal signal, but not a “viral” one. Whether the mechanism is electromagnetic, photonic, or something else entirely, the critical point remains: CPE alone is not proof of “viral” causation.
Remarkably, the CIA took notice of Kaznacheev’s research. In an unclassified translation of a Russian article from 1974, the author described how, when one of the cultures was contaminated with a “virus” or poisoned, “the most amazing thing happens: after the first culture has perished, it becomes the turn of the second culture, even though the possibility of the virus entering has been excluded.” The “mirror cytopathic effect” phenomenon was officially recognized as a scientific discovery and recorded in the USSR’s registry of discoveries as No. 122.
Designation 122 corresponds to a Soviet patent filed on February 15, 1966. It describes the “phenomenon of intra-cellular distanced electromagnetic interactions in a system of two tissue cultures.” The patent states:
“The present invention is registered on the former Soviet Union National Invention (Found) Register No. 122, published February 15, 1966 [Invention: V.P. Kaznacheev, S.P. Shurin, L.P. Mikhailova is based on the invention, "Phenomenon of intra-cellular distanced electromagnetic interactions in a system of two tissue cultures."
Another culture, manifested in the form of a "mirror" cytopathic effect (CPE) upon the action of a biological, chemical or physical property factor on either one of two identical cultures. Previously unknown phenomena of electromagnetic interactions between distant cells, with tissue specific responses, have now been established experimentally. Wherein the "mirror" cytopathic effect determines the cellular system as a detector for the regulating properties of electromagnetic radiation.’
As this artificial phenomenon is genuine and reproducible, it should raise serious questions about the longstanding assumption that cytopathic effects (CPE) in laboratory settings are inherently “viral” in origin. The appearance of similar degenerative changes in uninoculated cultures—under conditions free of any purported “virus”—suggested that cells may be responding to environmental stressors or even influencing each other through non-chemical, possibly electromagnetic means. This aligns with emerging interest in bioresonance and field-based models of cellular communication that challenge the conventional mechanistic paradigm. At the very least, such findings expose the fundamental unscientific nature of in vitro CPE studies and should cast serious doubt on their relevance to living organisms.
When virologists argue that a “virus” must exist because of the damage seen in a cell culture after inoculation with a clinical specimen, they are engaging in a form of circular reasoning. They assume the presence of a “virus,” introduce a sample presumed to contain it into a culture and then interpret any resulting cell death or morphological changes as evidence of the “virus’s” activity. This method not only begs the question—it also suffers from confounding, since numerous other factors could cause similar effects.
Cell cultures are sensitive systems, easily disturbed by a range of variables, including changes in pH, nutrient depletion, toxin accumulation, oxidative stress, mechanical agitation, and other stressors. If a culture shows CPE after inoculation, this could be due to any number of these factors—not necessarily the presence of a “pathogenic virus.”
In fact, the observation of CPE in both exposed and unexposed control cultures undermines the claim that CPE is a “virus-specific” effect. In a 1990 experiment that sought to replicate Kaznacheev’s “mirror cytopathic effect,” researchers irradiated chick embryo fibroblast cell colonies with ultraviolet (UV) light, resulting in a 30.1% cytopathic effect. Adjacent, unirradiated colonies placed in optical contact showed 28.8% cytopathy. Meanwhile, two control groups that received no UV exposure averaged 14.5% and 14.2% cytopathy, respectively. While the researchers assumed the increased CPE in the experimental groups was caused by UV exposure, this still leaves open the same fundamental question: were the effects truly the result of UV-induced intercellular signaling, or simply unaccounted-for environmental conditions?
Although the researchers confirmed that cells emit light after UV irradiation, they did not find compelling evidence that this emission led to structured, communicative effects in adjacent cells. They concluded:
“The mirror cytopathic effect reported by Kaznacheev was investigated to ascertain if light was emitted from chick embryo fibroblast tissue cultures after ultraviolet light radiation. The claim that these cells emit light after being irradiated by ultraviolet light was substantiated to our satisfaction. The mirror cytopathic effect was not. Other mechanisms for cell death in the cell colonies needs further investigation before Kaznacheev's claim can be accepted.”
While this undermined Kaznacheev’s interpretation of purposeful light-based communication, it did not eliminate the core observation—that similar cytopathic effects can appear in separate, non-inoculated cultures under shared conditions. The mechanism may remain unclear, but the implication is undeniable: CPE can arise through “non-viral,” possibly systemic causes.
Thus, even when dramatic CPE is observed, it does not validate the presence of a “virus.” It only shows that cell cultures can be damaged under a variety of artificial conditions. And if “uninfected” cultures also show CPE—or if similar effects can be induced through “non-viral” means like UV exposure—then the “logic” of equating CPE with “virus isolation” collapses.
This reveals that the appeal to CPE as virological evidence rests on a deeply flawed foundation. Without an independently verified cause—a true independent variable—the attribution of CPE to a “virus” remains an unproven assumption.
Crucially, these effects are only observed under artificial laboratory conditions: cells are removed from their native environment, deprived of normal inputs, and exposed to foreign substances, antibiotics, and often non-physiological media. These contrived circumstances do not mimic any natural biological context. As such, the degenerative effects they produce—including those labeled as “viral”—are artifacts of the experimental setup itself rather than signs of “pathogenic infection” and “viral replication.” The fact that similar effects can be induced without any “viral” agent at all indicates that what is being interpreted as “viral” pathology is a general stress response to toxic or unnatural conditions.
This opens the door to a dangerous subjectivity: once arbitrary and artificial effects are treated as meaningful signals, anyone can spin their own pet narrative to explain them. One researcher may claim a “novel virus;” another may claim “immune dysfunction;” a third may invoke “latent pathogens” or environmental triggers. But none of these are scientific conclusions—they are stories told about lab-created phenomena, without independent verification of any underlying cause. That is not science. It is storytelling masquerading as method.
Science, properly understood, is the disciplined observation of natural phenomena through testable, falsifiable hypotheses grounded in reality. Yet modern virology deviates from this standard. It constructs a closed system in which cellular breakdown is induced under contrived conditions and then retroactively attributed to a hypothetical “virus”—one that is neither isolated in the classical sense, nor directly observed causing disease in a living host. This inversion of method replaces direct empirical evidence with inference, and testable causation with interpretive correlation.
The cytopathic effect—and its so-called “mirror” in the mock CPE—are not windows into “viral” behavior in nature. They are experimental illusions: manufactured outcomes from systems designed to confirm prior assumptions. By treating these artificial responses as evidence of “viral” causation, virology shifts from science to simulation. It does not observe, it constructs. It does not discover, it presupposes. If science is to retain its integrity as a means of understanding the natural world, then virology must be held to the same empirical standards as any other discipline—or else be recognized for what it has become: a pseudoscientific detour grounded in circular reasoning, experimental artifacts, and a profound abandonment of the scientific method.
examined the real reasons why we get sick that do not include imaginary “viruses.”The Baileys also had a wide-ranging discussion with Etienne de la Boetie, the author of “Government” - The Biggest Scam in History, Exposed! and To See The Cage Is To Leave It - 25 Techniques the Few Use to Control The Many.
highlighted many sources stating that having measles (which is a detoxification process) is actually a good thing. applied a critical rethinking to measles as well. put the spotlight on RFK Jr.’s gutting of the Freedom of Information offices.She also shared that Finland’s National Institute for Health and Welfare (THL) can't cite scientific evidence of “H5N1” or any other “virus.”
investigated and dismantled the claims surrounding gonorrhea. focused on why the “virus” lie is a pivotal issue in the entire field of health care. had a revealing Q & A with author Roman Bystrianyk about the dramatic reduction in mortality from disease through sanitation, nutrition, and better living standards over vaccines.











Mike this is another really good article of yours. The Russian experiments, assuming I understood them adequately, provide more evidence for "resonance" between organisms. The "viral cytopathic effect" of course is long dead, buried, exhumed, and cremated. Funeral services were held decades ago. Unfortunately, virologists chose not to attend.
Thanks for linking my post, Mike, and thanks for the info on resonance. More and more that seems like the explanation for what looks like "contagion" when people get similar symptoms at the same time. I touch on this in my most recent article on chickenpox. Not everything can be explained with materialistic answers because we are not just materialistic beings, but energetic ones as well. Maybe someday the medical establishment will catch on (but I'm not going to hold my breath!). Thanks again for your top-notch research and writing. You totally rock!