Smelling a R.A.T.
The Rapid Antigen Tests are currently in use widely in order to determine "Covid" cases. Should they be?
While I have gone into great detail on the flaws and inaccuracies of both the PCR and antibody tests on viroLIEgy.com, I have not really done a deep dive yet on the rapid antigen tests. I am often asked my opinion on RAT's and to break them down as I have done for the other devices claiming detection of a “SARS-COV-2.” It should be fairly obvious that any and all tests which claim to detect a “virus” that has never been scientifically proven to exist are by definition fraudulent. No “SARS-COV-2” particles have ever been purified and isolated directly from the fluids of a sick host, biochemically characterized, and proven pathogenic in a natural way. Thus, no “virus” particles exist in order to calibrate and validate any of the tests said to detect the nonexistent “virus.”
However, even though I have not done an in-depth review of these tests yet, I have been collecting various bits of information on them over the years. As the RAT's are an often requested topic, I wanted to provide some of what I have come across here. What you will see is that, based upon information provided by official sources, RAT's are less accurate and reliable, not as sensitive as the PCR, prone to false-positive results, and rely on many factors as well as interpretation in order to be considered valid.
In January 2021, the CDC released a report on the Sofia SARS Antigen FIA, one of the rapid antigen tests released out on the Emergency Use Authorization. In this report, the RAT was compared to the RT-PCR and it was ultimately concluded that the RAT's were less accurate and in many instances, results needed to be verified by RT-PCR. It was noted that “accuracy” with asymptomatic patients was low at 41.2%. There were other revealing tidbits about RAT's within the report. Highlights follow:
"What is already known about this topic?
Antigen tests for SARS-CoV-2 are inexpensive and can return results within 15 minutes, but test performance data in asymptomatic and symptomatic persons are limited.
What is added by this report?
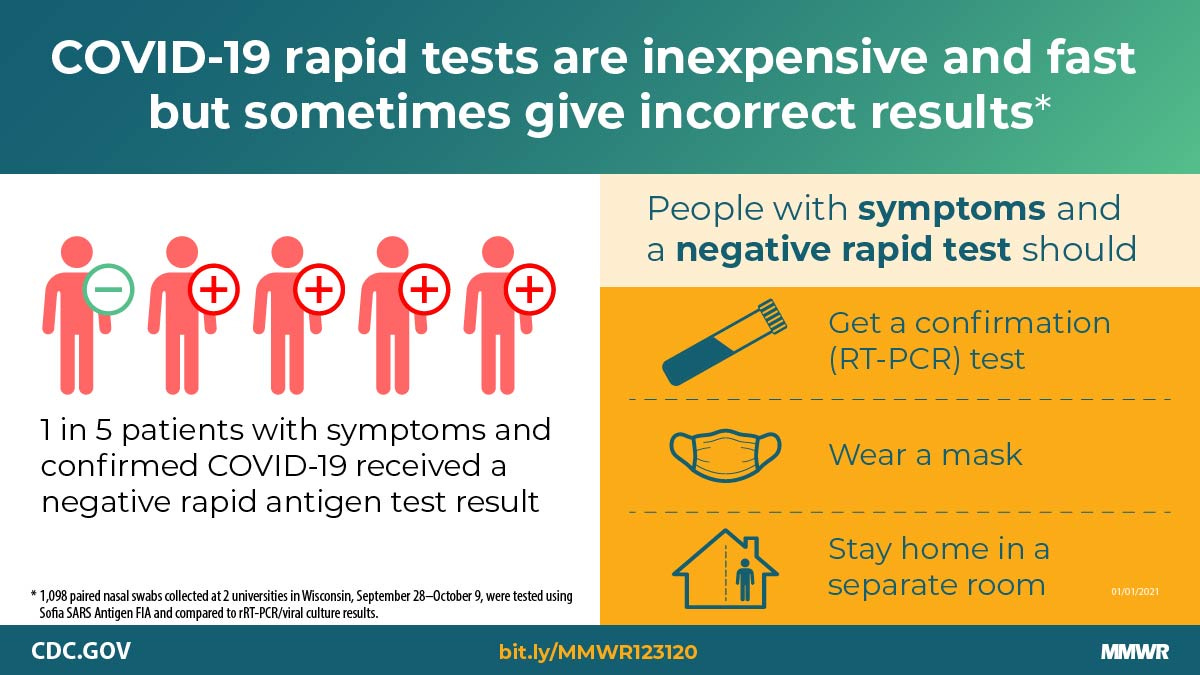
Compared with real-time reverse transcription–polymerase chain reaction (RT-PCR) testing, the Sofia antigen test had a sensitivity of 80.0% and specificity of 98.9% among symptomatic persons; accuracy was lower (sensitivity 41.2% and specificity 98.4%) when used for screening of asymptomatic persons.
What are the implications for public health practice?
To account for reduced antigen tests accuracy, confirmatory testing with a nucleic acid amplification test (e.g., RT-PCR) should be considered after negative antigen test results in symptomatic persons and positive antigen test results in asymptomatic persons."
"These tests have been used at U.S. colleges and universities and other congregate settings (e.g., nursing homes and correctional and detention facilities), where serial testing of asymptomatic persons might facilitate early case identification (3–5). However, test performance data from symptomatic and asymptomatic persons are limited."
"Virus culture was attempted on all antigen-positive or real-time RT-PCR–positive specimens. Among 871 (79%) paired swabs from asymptomatic participants, the antigen test sensitivity was 41.2%, specificity was 98.4%, and in this population the estimated positive predictive value (PPV) WAS 33.3%, and negative predictive value (NPV) was 98.8%. Antigen test performance was improved among 227 (21%) paired swabs from participants who reported one or more symptoms at specimen collection (sensitivity = 80.0%; specificity = 98.9%; PPV = 94.1%; NPV = 95.9%). Virus was isolated from 34 (46.6%) of 73 antigen-positive or real-time RT-PCR–positive nasal swab specimens, including two of 18 that were antigen-negative and real-time RT-PCR–positive (false-negatives). The advantages of antigen tests such as low cost and rapid turnaround might allow for rapid identification of infectious persons. However, these advantages need to be balanced against lower sensitivity and lower PPV, especially among asymptomatic persons. Confirmatory testing with an FDA-authorized nucleic acid amplification test (NAAT), such as RT-PCR, should be considered after negative antigen test results in symptomatic persons, and after positive antigen test results in asymptomatic persons (1)."
"At specimen collection, 227 (20.7%) participants reported experiencing one or more COVID-19 symptoms, AND 871 (79.3%) reported no symptoms."
"Among 227 paired specimens from symptomatic participants, 34 (15.0%) were antigen-positive, and 40 (17.6%) were real-time RT-PCR-positive."
"Sixteen paired swabs were antigen-positive and real-time RT-PCR–negative (i.e., false-positive), including 14 (66.7%) of 21 positive antigen results from asymptomatic participants and two (5.9%) of 34 from symptomatic participants."
"Second, given the limitations of RT-PCR, some false-positive antigen test results might represent true infections not identified by RT-PCR."
"Specimens were used to perform a limiting-dilution inoculation of Vero CCL-81 CELLS, and cultures showing evidence of cytopathic effect (CPE) were tested by real-time RT-PCR for the presence of SARS-CoV-2 RNA. Viral recovery was defined as any culture in which the first passage had an N1 Ct at least twofold lower than the corresponding clinical specimen."
"Virus was recovered from 34 (46.6%) of 73 positive specimens, including 32 (82.1%) of 39 specimens with concordant positive results and two (11.1%) of 18 with false-negative antigen results; no virus was recovered from 16 specimens with false-positive antigen test results. The two specimens with false-negative antigen results that were culture-positive were from two symptomatic participants who had specimens collected at day 2 and day 4 after symptom onset.Discussion
The Sofia SARS Antigen FIA received FDA EUA on May 8, 2020, for use in symptomatic persons within 5 days of symptom onset (2). In this investigation, among persons reporting COVID-19–compatible symptoms at specimen collection, the test was less accurate (sensitivity = 80.0%; specificity = 98.9%) than reported in the FDA EUA (sensitivity = 96.7%; specificity = 100%) (2). Two of eight specimens from symptomatic persons that had false-negative antigen test results were positive by viral culture, indicating that potentially infectious persons might not be detected by antigen testing. To reduce the impact of false-negative antigen test results, confirmatory testing with an FDA-authorized NAAT, such as RT-PCR, should be considered following negative antigen test results in symptomatic persons (1).
Among asymptomatic participants, antigen test sensitivity was 41.2%, specificity was 98.4%, and PPV in this population was 33.3%. This low PPV was observed despite a relatively high prevalence of SARS-CoV-2 in this population (5.2% prevalence overall; 2.0% among asymptomatic persons), suggesting that PPV could be even lower when using this antigen test among populations with lower expected SARS-CoV-2 prevalence. To account for false-positive results when using antigen tests for asymptomatic screening, confirmatory NAAT testing should be considered following positive antigen test results in asymptomatic persons, particularly when pretest probability of SARS-CoV-2 infection is low (1).
The findings in this report are subject to at least four limitations. First, participants were predominantly young adults in university settings where ongoing serial testing was being conducted. Antigen test performance might differ in other populations with different characteristics and testing schedules. Second, given the limitations of RT-PCR, some false-positive antigen test results might represent true infections not identified by RT-PCR. Third, the ability to recover infectious virus in culture is limited and decreases for specimens with higher Ct values (8); a lack of virus recovery by culture does not indicate that a person is not infectious. Finally, this investigation evaluated the Sofia SARS Antigen FIA, and cannot be generalized to other FDA-authorized SARS-CoV-2 antigen tests.
Serial testing of asymptomatic and symptomatic persons has been proposed for prevention and control of SARS-CoV-2 transmission (9,10) and is currently being implemented at U.S. colleges and universities and in other congregate settings (3–5). Despite reduced sensitivity compared with real-time RT-PCR, the use of antigen tests for serial testing in these settings, particularly when RT-PCR tests are not available or have a prolonged turnaround time, might still allow rapid identification of infectious persons and control of outbreaks (1). However, antigen-based testing strategies should account for the lower sensitivity and lower PPV when used for asymptomatic screening by considering confirmatory testing with an FDA-authorized NAAT, such as RT-PCR, after a positive antigen test result in an asymptomatic person. Confirmatory testing should also be considered following a negative antigen test result in a person experiencing COVID-19–compatible symptoms. All persons with negative antigen test results should continue to take measures to prevent SARS-CoV-2 transmission, including wearing a mask, reducing contact with nonhousehold members, and getting tested if they experience symptoms or have close contact with someone who has COVID-19.††† Symptomatic persons with negative antigen test results should continue to follow CDC guidance for persons who might have COVID-19, including staying home except to get medical care and protecting household members by staying in a separate room, wearing a mask indoors, washing hands often, and frequently disinfecting surfaces.”
https://www.cdc.gov/mmwr/volumes/69/wr/mm695152a3.htm
In Summary:
Antigen test performance data in symptomatic/asymptomatic individuals is limited
Antigen "accuracy" is lower in asymptomatic individuals
PCR should be used to confirm negative symptomatic results and positive asymptomatic results in antigen tests
"Virus" was only "isolated" from 34 of 73 samples - less than half!
Only 15-17.6% of the 227 symptomatic cases were positive
Given PCR limitations, some false-positive antigen results may in fact be "true" positives MISSED BY PCR
There was a low positive predictive value (PPV) despite a relatively high prevalence of “SARS-CoV-2” in the asymptomatic population
PPV could be even lower when using this antigen test among populations with lower expected “SARS-CoV-2” prevalence
Given the limitations of RT-PCR, some false-positive antigen test results might represent “true infections” not identified by RT-PCR
In conclusion, they want us to believe that PCR should be used to confirm antigen test results due to the inaccuracies related to RAT's, while false-positive antigen tests may be true positive cases missed by PCR due to the limitations of PCR. Sounds perfectly logical, right? Also, all persons with negative antigen test results should continue to take measures to prevent “SARS-CoV-2” transmission and symptomatic persons with negative antigen test results should continue to follow CDC guidance for persons who might have “COVID-19.” In other words, whatever the RAT result, act as if you have “Covid” as the test results are absolutely MEANINGLESS.
Interestingly, the Sofia Antigen test cited above was also found in another study to be creating a great deal of false-positive results:
While Quidel’s Sofia SARS Antigen FIA test produced more false positives than PCR-confirmed positives in the study, the company’s “intended use” document states that the diagnostic is for the detection of SARS-CoV-2 in individuals who are “suspected of COVID-19 by their healthcare provider within the first five days of the onset of symptoms.”
The accuracy of the RAT's was also put into question in December 2020 when the FDA released a letter to healthcare providers on the potential for high false-positive results from the tests:
Potential for False Positive Results with Antigen Tests for Rapid Detection of SARS-CoV-2 - Letter to Clinical Laboratory Staff and Health Care Providers
The U.S. Food and Drug Administration (FDA) is alerting clinical laboratory staff and health care providers that false positive results can occur with antigen tests, including when users do not follow the instructions for use of antigen tests for the rapid detection of SARS-CoV-2. Generally, antigen tests are indicated for the qualitative detection of SARS-CoV-2 antigens in authorized specimen types collected from individuals who are suspected of COVID-19 by their healthcare provider within a certain number of days of symptom onset. The FDA is aware of reports of false positive results associated with antigen tests used in nursing homes and other settings and continues to monitor and evaluate these reports and other available information about device safety and performance.
The FDA reminds clinical laboratory staff and health care providers about the risk of false positive results with all laboratory tests. Laboratories should expect some false positive results to occur even when very accurate tests are used for screening large populations with a low prevalence of infection. Health care providers and clinical laboratory staff can help ensure accurate reporting of test results by following the authorized instructions for use of a test and key steps in the testing process as recommended by the Centers for Disease Control and Prevention (CDC), including routine follow-up testing (reflex testing) with a molecular assay when appropriate, and by considering the expected occurrence of false positive results when interpreting test results in their patient populations.
Remember that positive predictive value (PPV) varies with disease prevalence when interpreting results from diagnostic tests. PPV is the percent of positive test results that are true positives. As disease prevalence decreases, the percent of test results that are false positives increase.
For example, a test with 98% specificity would have a PPV of just over 80% in a population with 10% prevalence, meaning 20 out of 100 positive results would be false positives.
The same test would only have a PPV of approximately 30% in a population with 1% prevalence, meaning 70 out of 100 positive results would be false positives. This means that, in a population with 1% prevalence, only 30% of individuals with positive test results actually have the disease.
At 0.1% prevalence, the PPV would only be 4%, meaning that 96 out of 100 positive results would be false positives.
Health care providers should take the local prevalence into consideration when interpreting diagnostic test results.
Like molecular tests, antigen tests are typically highly specific for the SARS-CoV-2 virus. However, all diagnostic tests may be subject to false positive results, especially in low prevalence settings. Health care providers should always carefully consider diagnostic test results in the context of all available clinical, diagnostic and epidemiological information. Test interference from patient-specific factors, such as the presence of human antibodies (for example, Rheumatoid Factor, or other non-specific antibodies) or highly viscous specimens could also lead to false positive results.
What the FDA wants you to believe is that false-positives are no big deal as they happen all the time, even in tests which are considered extremely accurate. However, notice the FDA's admission that disease prevalence affects the accuracy of the test results? This means that if disease prevalence is considered low, false-positive results will be high. Disease prevalence can only be determined by clinical diagnosis. This creates a bit of a problem as “Covid-19” can not be diagnosed clinically as the symptoms overlap with many other diseases.
“We showed that CoV infections are clinically diverse and, as also has been shown by earlier studies [16], cannot be diagnosed on the basis of clinical symptoms.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5986163/
Thus, the only way to determine disease prevalence is through testing to build cases. Yet test results can only be considered accurate if disease prevalence is known beforehand. Can you see the circular reasoning here? The test can not be used to build cases to establish disease prevalence in order to determine the accuracy of the test itself. I explained this problem in more detail here:
https://viroliegy.com/2021/09/30/pcr-and-the-prevalence-problem/
Rapid Tests Not so Specific…
We can get an even greater idea about the inaccuracy of RAT's by looking at some of the admitted faults regarding the tests. For example, the Clinitest Rapid "Covid" test is said to detect the "SARS-COV-2" antigens only:
"The CLINITEST® Rapid COVID-19 Antigen Self-Test is a lateral flow chromatographic immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2."
"A positive test result for COVID-19 indicates that antigens from SARS-CoV-2 were detected, and therefore the patient is infected with the virus and presumed to be contagious."
According to the manufacturer, this is how the test works:
"The CLINITEST Rapid COVID-19 Antigen Test is an immunochromatographic membrane assay that uses highly sensitive monoclonal antibodies to detect nucleocapsid protein from SARS-CoV-2 in direct nasopharyngeal (NP) swab or nasal swab. The test strip is composed of the following parts: namely sample pad, reagent pad, reaction membrane, and absorbing pad. The reagent pad contains the colloidal-gold conjugated with the monoclonal antibodies against the nucleocapsid protein of SARS-CoV-2; the reaction membrane contains the secondary antibodies for nucleocapsid protein of SARS-CoV-2. The whole strip is fixed inside a plastic device. When the sample is added into the sample well, conjugates dried in the reagent pad are dissolved and migrate along with the sample. If SARS-CoV-2 nucleocapsid antigen is present in the sample, a complex forms between the anti-SARS-2 conjugate and the virus will be captured by the specific anti-SARS-2 monoclonal antibodies coated on the test line region (T)."
Notice the use of the word SPECIFIC when referring to anti-SARS-COV-2 monoclonal antibodies? Maybe they should rethink the use that word as they later admit:
"Positive test results do not differentiate between SARS-CoV and SARS-CoV-2."
Interestingly, the same admission was made by the FDA here:
"The Rapid SARS-CoV-2 Antigen Test Card does not differentiate between SARS-CoV or SARS-CoV-2 viruses."
As well as in the BinaxNOW Rapid test:
"Positive test results do not differentiate between SARS-CoV and SARS-CoV-2."
According to the Roche "SARS-COV-2" Rapid Antigen Test, not only does the test cross-react with “SARS-COV-1” but also “coronavirus” HKU1 and Pneumocystis jirovecii:
"There was no cross-reaction and interference with the potential cross-reacting microorganisms listed below except SARS-CoV."
"Human coronavirus HKU1 and Pneumocystis jirovecii (PJP) have not been tested. There can be cross‑reaction with Human coronavirus HKU1 and Pneumocystis jirovecii (PJP), even though the % identity of the nucleocapsid protein sequence of HKU1 and PJP with the nucleocapsid protein sequence of SARS‑CoV‑2 was 35.22 % and 16.2 % which is considered as low homology."
With the INDICAID rapid test insert, we see similar admissions as well:
“In silico analysis was performed using the Basic Local Alignment Search Tool (BLAST) managed by the National Center for Biotechnology Information (NCBI) to estimate the likelihood of cross-reactivity with microorganisms not available for wet-testing. The degree of protein sequence homology was determined between the SARS-CoV-2 nucleocapsid protein antigen and the following microorganisms:
• Human Coronavirus HKU1: Sequence homology between SARS-CoV-2 nucleocapsid protein and Human Coronavirus HKU1 nucleocapsid protein is relatively low at 36.7% across 82.0% of sequences, but cross-reactivity can not be ruled out.
• Mycobacterium tuberculosis: No protein sequence homology was found between the SARS-CoV-2 nucleocapsid protein and Mycobacterium tuberculosis total protein (5925 sequences). Homology-based cross-reactivity can not be ruled out.
• Pneumocystis jirovecii (PJP): No protein sequence homology was found between the SARS-CoV-2 nucleocapsid protein and PJP total protein (3762 sequences). Homology-based cross-reactivity can not be ruled out.
• SARS Coronavirus: Sequence homology between SARS-CoV-2 nucleocapsid protein and SARS-Coronavirus nucleocapsid protein was found to be 90 .5% with 100 % query sequence coverage. Cross-reactivity with SARS Coronavirus can not be ruled out."
The "specific" antibodies used for these Rapid Antigen tests can not differentiate between proteins from two different "viruses." This means that people who are being diagnosed as “SARS-COV-2” patients may in fact be “SARS-COV-1” patients as the test can not tell the difference between the two. These tests are also admitted to potentially cross-react with “coronavirus” HKU1, pneumocystis jirovecii, and mycobacterium tuberculosis. So much for the supposed specificity of the test results. If that wasn't bad enough, RAT's also can not tell the difference between "viral" proteins, soft drinks, alcohol, and water:
"We observed that all soft drinks (Coca-Cola, Coca-Cola Zero, Fanta-Orange, Orange soft drink), energy drink (Red Bull), alcoholic beverages (vodka, whiskey, and brandy), commercially bottled mineral water, and carbonated mineral water caused the appearance of a red test line (Figure 1 )."
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8372450/
It is absolutely clear that the antibodies used for the antigen tests and the diagnosis which result from them are not so specific after all.
…And Not so Accurate.
However, if at first you don't succeed...keep on testing until you get the positive result that they want. Or at least, that is seemingly the new mantra from the FDA:
Concerns over accuracy of COVID rapid test results when first sick
"At-home coronavirus screening has become a way of life for many Californians, but some medical experts are now cautioning that one test may not be enough to definitively determine whether someone is infected."
"The U.S. Food and Drug Administration suggested last week that those checking to determine whether they are infected should use multiple tests over a period of days."
“When you perform an at-home COVID-19 antigen test and you get a positive result, the results are typically accurate,” government officials wrote in a public statement. “However, if you perform an at-home COVID-19 antigen test, you could get a false negative result.”
Because of this, the agency “recommends repeat testing following a negative result, whether or not you have COVID-19 symptoms.”
"Some people aren’t testing positive using rapid tests until four or five days after they start to show symptoms, according to UC San Francisco infectious diseases expert Dr. Peter Chin-Hong."
"If you have COVID-19 symptoms and get a negative rapid test result, the FDA suggests testing again 48 hours later. If the second test is negative and you’re still concerned your symptoms are caused by COVID-19, the FDA suggests either a third rapid test or a lab-based PCR test.
“The FDA is highlighting the continued need for repeat, or serial, testing when people get a negative result with an at-home COVID-19 antigen test, including recommending additional testing over a longer period of time.”
The Omicron variant family is generally more likely to begin infection in the throat, Chin-Hong said, meaning “it takes a while to go up to the nose.”
So, if you’re just swabbing your nose during a test “and you have a sore throat, it may mean that you’re not getting virus there yet,” he said.
Another possible reason is that the immune systems of people who have been vaccinated and boosted are more likely to recognize an exposure to the coronavirus quickly, triggering symptoms early as a way to fight off disease but before there are levels of virus in the body high enough for a rapid test to detect."
Before widespread vaccinations, the immune system took a relatively long time before recognizing the coronavirus and triggering an immune response.
But “in a vaccinated and boosted person, they already have immune cells floating around to recognize the enemy. And when you get infected, the immune system activates much faster,” Chin-Hong said. “Now, the immune system is saying, ‘Hey, something’s up!’ and you start to feel ill, but actually, there is not much virus around.”
What did we learn today?
Keep testing with the RAT until you get a positive result.
If these tests do not give you a positive result, get a PCR test.
If neither of these tests give you a positive result, still seclude yourself as if you are positive.
If you feel symptoms in your throat first but you are negative after swabbing your nose, the "virus" has not worked it's way up into your nose yet... 🤦♂️
For the vaccinated and boosted, symptoms mean that you do not have a lot of "virus" as your immune system recognized it early.
Any questions?
Bottom line: keep on testing with inaccurate tests until you get yourself a big fat positive.
You ever wonder why they don't recommend repeat serial testing to ensure that a positive result is accurate...? 🤔
Dr. Saeed Qureshi did a very good article recently on the fraudulent “Covid” tests which unfortunately got him banned on Facebook:
https://bioanalyticx.com/why-are-pcr-and-rapid-antigen-tests-false-and-fraudulent/
Dr. Kevin Corbett did an excellent presentation for the MEDICAL DOCTORS FOR COVID ETHICS INTERNATIONAL
on October 5th, 2022
https://rumble.com/v1nf8og-kevin-corbett.html
Dr. Mark Bailey was on the Jerm Warfare podcast doing what he does best and demolishing all things virology:
https://tntradiolive.podbean.com/e/dr-mark-bailey-on-jerm-warfare-with-jeremy-nell-03-october-2022/
Mark was also smelling a R.A.T. back in March 2022 when he wrote this brilliant article calling out the Testing Pandemic:
https://drsambailey.com/rapid-antigen-tests-making-viruses-real-again/
Not to be outdone by her other half, Dr. Sam Bailey put out two brilliant videos, one breaking down the bioweapons scam and the other an eye-opening interview with German engineer Marvin Haberland and his work to get the German courts to publicly admit that “SARS-COV-2” does not exist:
https://drsambailey.com/resources/videos/viruses-unplugged/bioweapons-bs/
https://drsambailey.com/resources/videos/interviews/marvin-vs-virology-covid-taken-to-court/







I just declined an invitation to a Spiral Dance, "an annual event honoring the dead and welcoming the New Year. Intention: to weave a web of connection that strengthens our communities, heals our griefs, and empowers Justice to prevail." Cool, right? Except that they require a RAT to participate. Spit Test Wiccans.
Thanks, Mike. Imagine a test so inaccurate that it needs to be "confirmed" by checking with the highly inaccurate PCR test, which itself has never been calibrated with an actual virus.