It looks like it is time once again for yet another Ebola panic. Do you have your trusty hazmat suit and related personal protective gear at the ready in case the “virus” decides for a cross-continental getaway from Africa? After the recent Marburg outbreak in Ghana a few months ago, you had to have known this was coming, right? Wherever Marburg is, its “cousin” never seems to be too far behind.
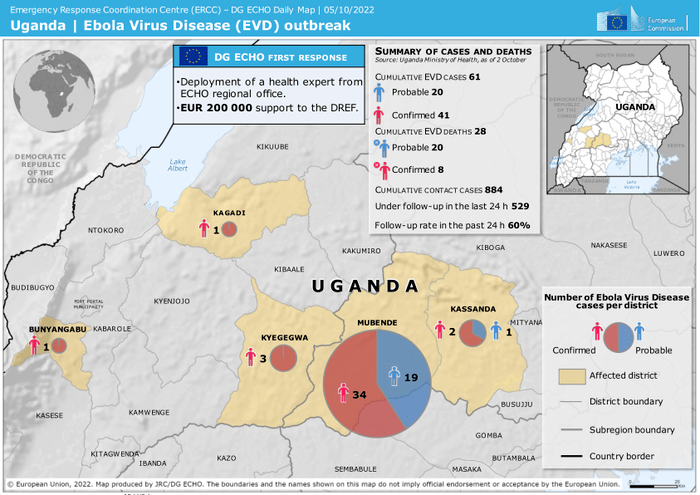
Currently, Uganda is being terrorized by the “rare and deadly” Ebola with the estimates ranging from about 50 to 64 people “infected”with anywhere from 19 to 30 fatalities, depending on the source. If for some reason you believe the news, it seems that the time is upon us to cower in fear of this “virus” as the strain making the rounds, the “Sudan strain,” has no vaccines nor treatments effective against it. Fortunately for those who have generated the appropriate fear response from the aforementioned news, there are six candidate vaccines in the pipeline, with the furthest along being developed by the very “trustworthy” NIAID organization run by Anthony Fauci. However, if you did just now release a sigh of relief at the promising thought of a Sudan-specific vaccine and antivirals, don't relax your guard just yet. Even though previous “outbreaks” have come and gone without the use of these experimental treatments, we are told that, while necessary for some reason, these treatments are not the magic bullets that will stop the Sudanese Ebola in its tracks:
Ebola outbreak in Uganda: how worried are researchers?
The outbreak has already spread to five districts, and there are no proven vaccines for this species of the virus.
“In the past month, at least 64 people in Uganda have been — or are suspected of having beeen — infected with a rare species of the Ebola virus, for which no vaccines or treatments are available. About 30 people have died. The rapid rise and spread of the lethal virus across five districts in Uganda have alarmed scientists, and raised fears that the outbreak will not be easy to contain.”
“Ebola is a rare and deadly disease — with a death rate that has ranged from 25% to 90% in past outbreaks. The first symptoms to develop are usually fever, vomiting, headaches and fatigue, but the condition can worsen quickly to include damage to internal organs and death.
Two main viral species give rise to Ebola in humans: Zaire ebolavirus and Sudan ebolavirus. Zaire ebolavirus caused a large epidemic from 2013 to 2016 in West Africa that spurred the development of vaccines and treatments, which have since transformed the fight against Ebola. But similar therapies for Sudan ebolavirus — responsible for the current outbreak in Uganda — are still in clinical trials. The most recent outbreak caused by this species occurred in 2012 in Uganda.”
“Although Sudan ebolavirus had not been known to cause a human infection in about a decade, it was only a matter of time before it resurfaced, says Kartik Chandran, a virologist at the Albert Einstein College of Medicine in New York City. “These viruses are out there,” he says, “and we don’t have a good handle of where they hang out in nature, and how they transmit to people.” Ebola outbreaks have been difficult to prevent, because animals such as some monkeys and bats can carry the viruses and spread them to people. In rare cases, the viruses can linger silently in a person’s body for months or even years after an initial infection1, only to emerge later and spread to others.
Vaccines are not enough
“Because Sudan ebolavirus outbreaks have been rare, researchers have not been able to test vaccine candidates thoroughly. Three vaccines have undergone early tests to ensure that they are safe in humans, but the larger trials needed to confirm efficacy haven’t been possible.
There are currently six candidates in the pipeline. The one that is furthest along is a single-dose vaccine that was developed in part by the US National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland, and is licensed to the Sabin Vaccine Institute in Washington DC. A study has shown that this jab is protective against the Sudan species in non-human primates2. One hundred doses might be shipped from NIAID to Uganda as early as next week, says Richard Koup, the acting director of NIAID’s Vaccine Research Center.
These should be prioritized for hospital workers, including health-care personnel who are interacting with people who have been infected and their direct contacts, and the contacts of those contacts, says Gary Kobinger, a virologist at the University of Texas Medical Branch in Galveston who specializes in Ebola. Still, vaccines and antivirals, even if proved to be effective, will not stop the outbreak just because they exist, Kobinger says. To achieve that, enough doses need to be produced quickly and then distributed widely, which will pose a challenge, he adds.”
“Braka urges countries bordering Uganda to have Ebola test kits ready to distribute, and to ramp up surveillance for the virus. Countries outside Africa are also on alert. The US Centers for Disease Control and Prevention announced on 6 October that the United States will redirect travellers coming from Uganda to one of five US airports that are able to screen for the virus.”
https://www.nature.com/articles/d41586-022-03192-8

So how does one go about stopping a “virus” without magic bullets? The answer is obviously magic tests. According to the experts, the rapid diagnosis of the Ebola “virus” is the best way to thwart its evil plans for a world domination tour. However, how does one rapidly diagnose a disease where the associated symptoms overlap with numerous other diseases? How would a clinician be able to distinguish between influenza, “Covid-19,” and Ebola as they all share the same symptoms of disease? In fact, the symptoms of pregnancy are even said to mimic Ebola. How does a woman determine whether her morning sickness is the blessing of a child forming inside of her womb versus a sneaky stealth attack by an invisible invader? Worse still, how does one determine whether they are hiccuping due to the consumption of a deliciously spicy meal versus facing the emergence of a deadly pathogen hijacking the cells of their body?
Signs and Symptoms
“Primary signs and symptoms of Ebola often include some or several of the following:
Fever
Aches and pains, such as severe headache and muscle and joint pain
Weakness and fatigue
Sore throat
Loss of appetite
Gastrointestinal symptoms including abdominal pain, diarrhea, and vomiting
Unexplained hemorrhaging, bleeding or bruising
Other symptoms may include red eyes, skin rash, and hiccups (late-stage).
Many common illnesses can have the same symptoms as EVD, including influenza (flu), malaria, or typhoid fever.”
https://www.cdc.gov/vhf/ebola/symptoms/index.html
As differential diagnosis is impossible based upon the non-specific clinical symptoms alone, the only way to be able to rapidly label someone with the scarlet letter that is Ebola is through the oft-repeated “Covid” mantra of testing, testing, and more testing. The only “test” suitable for the task is the polymerase chain reaction (PCR) method used and abused throughout the “Covid pandemic:”
Diagnosis
“Diagnosing Ebola virus disease (EVD) shortly after infection can be difficult. Early symptoms of EVD such as fever, headache, and weakness are not specific to Ebola virus infection and often are seen in patients with other more common diseases, like malaria and typhoid fever.”
“Polymerase chain reaction (PCR) is one of the most commonly used diagnostic methods because of its ability to detect low levels of Ebola virus. PCR methods can detect the presence of a few virus particles in small amounts of blood, but the ability to detect the virus increases as the amount of virus increases during an active infection. When the virus is no longer present in great enough numbers in a patient’s blood, PCR methods will no longer be effective. Other methods, based on the detection of antibodies an EVD case produces to an infection, can then be used to confirm a patient’s exposure and infection by Ebola virus.”
https://www.cdc.gov/vhf/ebola/diagnosis/index.html
As with the “Covid” PCR tests, the same drawbacks, disadvantages, and lack of FDA-approval applies to those utilized for Ebola. According to their own information, using PCR to detect Ebola RNA only means that one is presumptively an Ebola case and that they are presumed to be contagious. This is due to the fact that PCR can not detect any “virus,” only small fragments of RNA said to belong to one. PCR can also not tell if the patients are potentially infectious nor whether they are currently infected. Getting a negative result does not mean the patient is Ebola-free either so keep on testing for the extremely rare “virus” just to be sure.
One of the main reasons for the admitted inaccuracies of the PCR test is the issue in determining disease prevalence. In order for a PCR result to be interpreted “accurately,” disease prevalence (the proportion of a population who have a specific characteristic in a given time period) needs to be known. However, disease prevalence can only be determined based upon cases identified by clinical diagnosis of the symptoms. Ebola, like every other disease, is associated with non-specific symptoms that overlap with numerous other diseases and conditions. Therefore, clinical diagnosis is impossible and so the PCR tests are used to determine cases. Thus we run into the circular problem where the PCR test is used to generate cases in order to determine the disease prevalence estimates needed to interpret the PCR test results accurately. For more on this circular nonsense, please see this article I did on the PCR prevalence problem.
While the PCR tests are fraudulent, the “tests” like the EBOV NP rRT-PCR Assay were said to be designed to minimize (not eliminate) the likelihood of false positive (and negative) test results. However, the Ebola PCR tests are only allowed based on an extended Emergency Use Authorization. For those unfamiliar, this means that it has not been stringently tested to ensure its accuracy. The Ebola PCR tests are out there because there are no better alternatives:
CDC - Ebola Virus NP Real-Time RT-PCR Assay
What does it mean if the specimen tests positive for Ebola virus?
"A positive test result for Ebola virus indicates that RNA from Ebola virus was detected, and the patient is presumptively infected with Ebola virus (species Zaire ebolavirus) and presumed to be contagious. Laboratory test results should always be considered in the context of clinical observations and epidemiological data in making a final diagnosis and patient management decisions. Patient management should follow current CDC guidelines.
The EBOV NP rRT-PCR Assay has been designed to minimize the likelihood of false positive test results. However, in the event of a false positive result, risks to patients could include the following: a recommendation for quarantine of the patient, monitoring of household or other close contacts for symptoms, patient isolation that might limit contact with family or friends and may increase contact with other potentially EVD infected patients, the ability to work, the delayed diagnosis and treatment for the true infection causing the symptoms, unnecessary prescription of a treatment or therapy, or other unintended adverse effects."
What is an EUA?
"The United States (U.S.) FDA has made this test available under an emergency access mechanism called an Emergency Use Authorization (EUA). The EUA is supported by a Secretary of Health and Human Service’s (HHS’s) declaration that circumstances exist to justify the use of in vitro diagnostic devices (IVDs) under EUA for the detection of Ebola virus."
This Ebola Emergency Use Authorization was established back in 2014 based on the presumed national security threat of an Ebola outbreak. By using the EUA, manufacturers can get around the more stringent testing and quality control requirements needed to be satisfied in order to get full FDA approval. As far as I can tell, the EUA is still in effect today and, just like with “Covid-19,” there are ZERO FDA-approved Ebola tests and treatments currently available:
2014 Ebola Virus Emergency Use Authorizations
“On September 22, 2006, then-Secretary of the Department of Homeland Security (DHS), Michael Chertoff, determined, pursuant to section 319F-2 of the Public Health Service (PHS) Act (42 U.S.C. § 247d-6b), that the Ebola virus presents a material threat against the United States population sufficient to affect national security. Pursuant to section 564(b)(1) of the Act (21 U.S.C. § 360bbb-3(b)(1)), and on the basis of such determination, the Secretary of HHS declared on August 5, 2014, that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection of Ebola virus, subject to the terms of any authorization issued under 21 U.S.C. § 360bbb-3(a).”
Here is the actual EUA declaration with no end date listed:
The Authorization is effective as of August 5, 2014.
Thus, it appears that manufacturers have been given considerable leeway to bypass normal FDA regulations and quality controls in order to put to the market products which lack proper quality control and verification/validation as quickly as possible. As expected, this leads to variable quality:
“The Ebola assays authorized by the FDA vary widely in terms of gene targets, performance, speed, and most notable, ease of use. The stated limits of detection of the Ebola assays listed by the FDA vary hugely, from 0.13 PFU/ml to 6 × 105 PFU/ml (see Table 1) although methodologic differences, both in preparation of reference material and manner of testing, make these numbers difficult to assess.”
https://www.tandfonline.com/doi/full/10.1586/14737159.2015.1077117
This Ebola “emergency” has now been going on for 8 years with no apparent end in sight. Whereas the outbreaks used to sporadically occur, they are now becoming an annual thing. Since the EUA declaration in 2014, there was a year off in 2016 and since then, there have been yearly outbreaks from 2017 to 2022. As with “Covid,” they can keep this emergency ongoing so that none of the tests and treatments ever need to meet the non-emergency regulatory standards.
Regardless of whether the tests are '“FDA approved” or only out on EUA, not a single one used can be considered accurate as there is no actual gold standard, i.e. purified and isolated Ebola “viral” particles, to calibrate and validate the tests against. The previous “gold standard” was “virus isolation” via cell culture. However, cell culture is not true isolation as many foreign elements and toxic chemicals are added together, the exact opposite of isolation. The only way to properly calibrate and validate the “tests” would be to properly purify and isolate the particles claimed to be Ebola directly from the fluids of a sick patient yet this is admitted to be an impossibility. Thus, the original “gold standard” was not a true gold standard and can not be used to evaluate and elevate PCR as the new “gold standard.” Nonetheless, PCR is now considered the “gold standard” despite the wide variance in testing outcomes:
“But Pollock admits the numbers may be misleading. After the collection of the initial data, the scientists used a second PCR-based test to look at some of the samples. The PCR test they'd been using as a "gold standard," it turned out, wasn't itself 100% sensitive, not only highlighting the discrepancy between different PCR-based tests but also casting doubts on their new results. In retrospect, "we think we probably overestimated the sensitivity and underestimated the specificity," Pollock says. More data are needed to illuminate these numbers, she adds.”
https://www.science.org/content/article/fingerprick-test-quickly-diagnoses-ebola
The discrepancies in the new “gold standard” were further highlighted in this 2016 review such as limited data on the specificity amd sensitivity of the tests, massive variations in the limits of detections between tests, varying performance between laboratory tests, reliable results being contingent upon appropriate sample handling and cross-contamination avoidance, and the lack of regulatory approval. It is also stated that any presumptive positive result must be “confirmed” by the CDC based upon their own fraudulent PCR test. Thus, one inaccurate test is being used to claim the reliability of the results generated by another inaccurate test:
Diagnosis of Ebola Virus Disease: Past, Present, and Future
“Compared to conventional RT-PCR, real-time amplicon detection using sequence-specific probes offers greater specificity and more rapid results (typically 2 to 3 h); however, limited data are available regarding the sensitivity and specificity of the various laboratory-developed and commercial Ebola virus real-time RT-PCR assays now employed by public health reference laboratories (25). Only one study to date has compared the analytic characteristics of commercially available Ebola virus real-time RT-PCR assays, and it demonstrated up to 100-fold variations in the limits of detection and 1,000-fold variations in the lower limits of quantitation (40).”
“The performance of these RT-PCR-based assays has been found to vary even among national reference laboratories (41), and reliable results are contingent upon both appropriate sample handling prior to analysis (to avoid RNA degradation) and avoidance of cross-contamination; thus, careful oversight and quality assurance measures are necessary to ensure adequate sample integrity and assay performance in field laboratory settings. Furthermore, assay design must take into account the potential for false-negative results due to PCR inhibitors present across specimen types, as well as sequence variation in novel virus strains/species.”
“Furthermore, although laboratory-developed Ebola virus RT-PCR assays were in routine use at multiple national reference laboratories, no EVD diagnostic tests had regulatory approval for clinical use at the beginning of the outbreak. To address these issues, the WHO set forth guidelines for laboratory diagnosis of EVD to promote standardization of biosafety and quality control measures (38, 50) and initiated an Emergency Use Assessment and Listing (EUAL) process for EVD diagnostic tests (51). The FDA also evaluated EVD diagnostic tests for issuance of Emergency Use Authorization (EUA) status.”
“Of note, all tests with FDA EUA status are approved for “presumptive” testing only; any positive presumptive Ebola virus test in the United States must be confirmed at the CDC (when performed at the CDC, the CDC Ebola virus NP and VP40 RT-PCR assays may currently be used for confirmatory testing, although a combination of testing modalities may be employed) (54).”
https://journals.asm.org/doi/10.1128/CMR.00003-16
As there are numerous issues with the current “gold standard” PCR test, researchers have become frustrated by the lack of a more accurate test to diagnose Ebola:
Researchers frustrated by failure to roll out 'game-changing' Ebola test
“Moreover, the independent validation has raised questions over the quality of some of the PCR testing that was done during the epidemic. In the study, researchers used a routinely employed, partly-automated PCR kit made by altona Diagnostics, of Hamburg, Germany. But some of the samples that the altona test scored as negative — and which the Corgenix kit scored as positive — were later confirmed to be positive using a reference-standard test kit. That is a concern, says Bhadelia, as false negatives by the altona test might have allowed people with Ebola to return to the community while they were still infectious. It also suggests the possibility that the Corgenix kit might not be as prone to false positives as Perkins suggests — though further research would be needed to clarify the kit's performance.”
https://www.nature.com/articles/nature.2015.17862
It has become apparent that researchers are beginning to turn their backs on the PCR test as the “gold standard” and are looking for other alternatives. As the current “gold standard” test is too inaccurate and too slow, it appears that they have decided that the best idea is to create and rely upon faster tests. However, as rapid tests are calibrated and validated against the PCR test, they are just as inaccurate. This variance in PCR results has led to frustration amongst researchers yet they somehow still believe that the “game-changing” test, one that can rapidly diagnose a disease before symptoms appear, is just waiting to be unleashed. However to date, despite the claims made about asymptomatic cases of disease, the ability to diagnose someone without symptoms for Ebola, influenza, and respiratory diseases does not exist:
Diagnosing Ebola: Why isn't there a rapid, reliable test?
“Complicating matters is the fact that the initial symptoms of Ebola -- high fever, headache, diarrhea, vomiting -- can seem much like those of other infectious diseases, including influenza. And with another U.S. flu season set to begin, how will worried Americans quickly know if they have that common bug -- or Ebola?”
A test that can tell the difference in minutes or even a few hours just isn't available right now, experts say.
First of all, there is no test at all to determine Ebola infection in a person without symptoms.
"Ebola has an incubation period of from two to 21 days, and nothing we have is effective at picking up infection before that happens," explained Philip Tierno, a clinical professor of microbiology and pathology at NYU Langone Medical Center in New York City.
"In fact, pre-symptomatic diagnosis is really a holy grail for infectious disease," added Dr. Amesh Adalja, a spokesman for the Infectious Disease Society of America. "It would be great to have it for Ebola and influenza, and a whole host of other infectious diseases, so we could intervene fast. And it's certainly something that many people are researching. But that kind of screening ability is really still in its infancy."
But what about after symptoms begin to appear, as in Duncan's case? Again, no speedy test for Ebola yet exists.
That means that "it's going to be very difficult to distinguish influenza from Ebola," Adalja said.
"Right now," Tierno added, "there's just no simple, fast Ebola test because there's been no demand. Until now, nobody has wanted to spend the money to get a commercially available rapid test out there."
https://www.cbsnews.com/news/diagnosing-ebola-why-isnt-there-a-rapid-reliable-test/
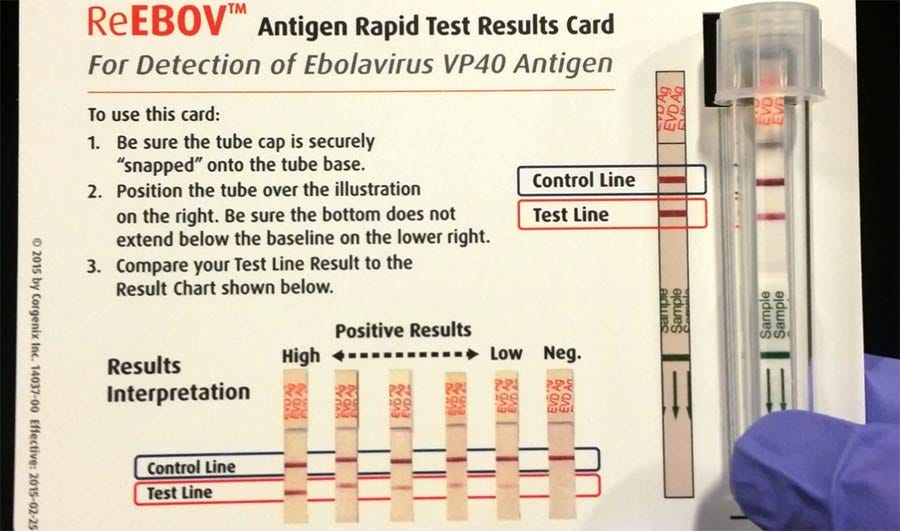
The “game-changing” rapid tests that are currently out on EUA vary in quality when tested against PCR, which itself varies in quality. Since 1976, none of the RATs released have been evaluated properly for effectiveness with clinical samples. In fact, there are no rapid tests that meet the sensitivity and specificity required by the WHO. None of the RATs could detect all of the suspected cases of Ebola. Upon testing the RATs against PCR, the results varied according to which “gold standard” PCR test was used to evaluate the RATs:
Rapid diagnostic tests versus RT–PCR for Ebola virus infections: a systematic review and meta-analysis
“Our search identified 113 unique studies, of which nine met the inclusion criteria. The studies were conducted in the Democratic Republic of the Congo, Guinea, Liberia and Sierra Leone and they evaluated 12 rapid diagnostic tests. We included eight studies in the meta-analysis. The pooled sensitivity and specificity of the rapid tests were 86% (95% confidence interval, CI: 80–91) and 95% (95% CI: 91–97), respectively. However, pooled sensitivity decreased to 83% (95% CI: 77–88) after removing outliers. Pooled sensitivity increased to 90% (95% CI: 82–94) when analysis was restricted to studies using the RT–PCR from altona Diagnostics as gold standard. Pooled sensitivity increased to 99% (95% CI: 67–100) when the analysis was restricted to studies using whole or capillary blood specimens.
Conclusion
The included rapid diagnostic tests did not detect all the Ebola virus disease cases. While the sensitivity and specificity of these tests are moderate, they are still valuable tools, especially useful for triage and detecting Ebola virus in remote areas.”
“Diagnosing the disease on these symptoms alone is challenging, because they are similar to common endemic diseases present in Africa, such as typhoid fever, malaria and yellow fever.3,4
To confirm the diagnosis of Ebola virus disease, a positive result from a reverse transcription polymerase chain reaction (RT–PCR) test is required.5 However, lateral flow assays (that is, rapid diagnostic tests) are valuable tools to limit the spread of the disease since their fast turnaround time has the potential to trigger early outbreak alerts.”
“According to the World Health Organization (WHO), rapid diagnostic tests for Ebola virus should have a desired clinical sensitivity of > 98% and an acceptable clinical sensitivity of more than 95%.9 Since 1976, many Ebola virus rapid diagnostic tests have been developed, but researchers have not yet thoroughly assessed the evidence of their performance in clinical samples. The few rapid diagnostic tests that have been assessed in field conditions demonstrated uncertainty and variability in performance.6”
“In this study, we conducted meta-analyses on clinical accuracy studies to assess the performance of rapid diagnostic tests to detect Ebola virus in individuals suspected of having the disease. Compared with the gold standard RT–PCR, the overall pooled sensitivity for rapid tests was 86%, falling short of a desired sensitivity of > 98% and an acceptable sensitivity of > 95% listed in a WHO target product profile document released during the 2013–2016 Ebola virus disease outbreak.9 Furthermore, we show that the overall pooled specificity was 95%, also lower than WHO’s recommended level of > 99%.9”
“Using a different gold standard also affected the results. The six data points with higher sensitivity all used altona as the gold standard.15,17,22,23 We also noted that pooled sensitivity was higher in studies using altona (90%) compared with other gold standards (78%). However, only six data points were available for other gold standards versus 13 for altona. It is unclear why using altona yielded a sensitivity superior to that of other gold standards. Reduced sensitivity of altona to specimens with cycle threshold values above 30 (i.e. low viral loads) have been observed.5,41 This reduced sensitivity of altona may affect the interpretation of our pooled estimates. Our pooled sensitivity might have been underestimated, since altona may fail to detect positive samples with low viral loads. However, we cannot rule out the possibility that studies using altona might also overestimate the sensitivity of rapid tests, since one study that used altona reported an unusual sensitivity of 100%.22”
“In conclusion, the results from this meta-analysis suggest that currently there is no commercial rapid diagnostic test for Ebola virus disease that has sufficient sensitivity and specificity to meet WHO standards.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9243686/#__ffn_sectitl
It should be fairly obvious now that the Ebola “virus” is a prime candidate to be the next breakout star. As with “SARS-COV-2,” we have a “virus” that was never properly purified nor isolated directly from the fluids of sick patients nor proven pathogenic in a natural way. We have a disease with associated symptoms which overlap with numerous other diseases and conditions making clinical diagnosis impossible. We have inaccurate PCR tests elevated to the “gold standard” in order to be used to generate cases to instill panic and fear as well as to confirm its own “accuracy.” We have a push to create less “accurate” rapid tests to generate even more cases as quickly as possible in order to diagnose those without disease as quickly as possible. We have experimental vaccines that have supposedly been in development behind the scenes ready for the limelight. We have tests and treatments that have never met the “high standards” of the FDA and WHO approval processes and are currently only authorized for use by an extended Emergency Use Authorization that remains in place indefinitely. We have all of the ingredients necessary for the direct sequel to the Testing Pandemic. Hopefully this is the sequel that no one shows up to see.
For a complete breakdown of the Ebola scam, please see these recent articles I did on viroLIEgy.com:
https://viroliegy.com/2022/08/29/the-marburg-virus-precursor-to-ebola/
https://viroliegy.com/2022/09/26/the-ebola-virus-part-1/
https://viroliegy.com/2022/10/03/the-ebola-virus-part-2/
Dr. Mark Bailey just released an excellent article describing the fraudulent practices used to produce “cases.” please check it out and support his and Dr. Sam Bailey's amazing work:
https://drsambailey.com/why-nobody-had-caught-or-got-covid-19/











Great work. Your research and presentation are world class.
Great stuff per usual Mike, Thank you!
I think Dustin Hoffman's protective suit failed him (lost the right-footsie-lost his "Tootsie")